Abstract
Ideally, public health policies are formulated from scientific data; however, policy-specific data are often unavailable. Big data can generate ecologically-valid, high-quality scientific evidence, and therefore has the potential to change how public health policies are formulated. Here, we discuss the use of big data for developing evidence-based hearing health policies, using data collected and analyzed with a research prototype of a data repository known as EVOTION (EVidence-based management of hearing impairments: public health pOlicy-making based on fusing big data analytics and simulaTION), to illustrate our points. Data in the repository consist of audiometric clinical data, prospective real-world data collected from hearing aids and an app, and responses to questionnaires collected for research purposes. To date, we have used the platform and a synthetic dataset to model the estimated risk of noise-induced hearing loss and have shown novel evidence of ways in which external factors influence hearing aid usage patterns. We contend that this research prototype data repository illustrates the value of using big data for policy-making by providing high-quality evidence that could be used to formulate and evaluate the impact of hearing health care policies.
Keywords: Big data, Hearing health care, Population health, Public health policy
The purpose of this article is to discuss the use of big data to support public health policy-making using an ongoing study as an illustrative example. This study, referred to as EVOTION (EVidence-based management of hearing impairments: public health pOlicy-making based on fusing big data analytics and simulaTION), aims to build the evidence base for the formulation of public health policies related to the prevention, early diagnosis, long-term treatment, and rehabilitation of hearing loss (HL). Its ultimate objective is to enable and support more holistic management of HL at the population level (Spanoudakis et al. 2018). As such, the study protocol has been published (Dritsakis et al. 2018), as have papers detailing data collection processes (Pontoppidan et al. 2017), the data repository architecture (Prasinos et al. 2017), decision modeling (Katrakazas et al. 2017) and approaches to data analysis (Christensen et al. 2019a). In this article, we expand on the discussion of Gutenberg et al. (2018) regarding the use of big data for developing evidence-based hearing health policies, using data collected and analyzed with a research prototype of the EVOTION decision-making platform to illustrate our points.
CURRENT PRACTICE IN HEALTH POLICY-MAKING
Demographic changes in populations due to increasing life span and the consequent increasing burden of chronic or age-related diseases have put pressure on governments to provide effective support and sustainable solutions to public health care issues (Lee 2003; Kehler 2019); however, because resources are limited, it is all the more important that they are distributed based on data-driven policies rather than on political and social agendas.
In general, the term “policy” describes a course of action, a roadmap, or a process that specifies how decisions are made and plans are carried out, so that goals within a society can be reached (World Health Organization 2019). Policies can inform and educate the population about health issues, ensure access to services, set goals for health services, and develop performance guidelines and standards (Institute of Medicine 1988). Policies are ideally constructed based on supporting scientific evidence. However, evidence-based policy-making is often suboptimal because scientific data that directly apply to the policy of interest are often unavailable. Further, when data are available, findings are often inconsistent, effect sizes are imprecise, variable measured are noncomparable, and/or there is evidence of publication bias (Orton et al. 2011; Oliver et al. 2014). As a result, policy-making processes are frequently steered by social contexts, political priorities, individual beliefs and preferences, social values, and the media (Larsen et al. 2012; Greer et al. 2017; van de Goor et al. 2017).
Like many others (Hernandez & Zhang 2017; Benke & Benke 2018; Mooney & Pejaver 2018), we contend that applying predictive analytics, machine learning, and statistical modeling to retrospective data combined with real-time prospective data collected on an ongoing basis from large numbers of people has the potential to change the status quo of policy-making. However, to date, little empirical evidence is available supporting this—the majority of publications discussing the application, advantages, and challenges of using big data in a public health context are merely hypothetical. Nonetheless, there are several studies that do illustrate how big data can be used for health policy-making purposes, as follows. Research has shown relationships between social media activity and new cases of infectious diseases (Young et al. 2014; Deiner et al. 2016), as well as relationships between geographically specific sentiments expressed via tweets about vaccinations and The Centers for Disease Control and Prevention-estimated vaccination rates by region (Salathé & Khandelwal 2011). This suggests that information from social media can potentially provide rapid warning about the spread of disease and/or content of needed public health campaigns. Aiming to improve cardiovascular health and delivery of health services, Tu et al. (2015) used clinical databases that held information about cardiovascular health and care in Ontario, Canada. The databases included electronic surveys, health administration, clinical, laboratory, drug, and electronic medical record databases. The study showed relationships between neighborhood walkability and air pollution and the predicted risk for hypertension and diabetes (Howell et al. 2019). Such findings have direct application to the development of public health policy. A third example of policy development from big data is the use of the US Veterans Health Administration’s electronic health record system for identifying and tracking complications from diabetes so that clinicians can be informed about patients at high risk from these complications, and care can better be coordinated (Luo et al. 2016).
These approaches could be applied to hearing healthcare, for instance, social media activity could be used to reveal audiological issues of concern among the general public so that public health campaigns about hearing awareness and activity could be developed; large scale real-world data about the physical and mental consequences of noise pollution could lead to policies about noise control and abatement; and electronic health records could be used to identify patients at risk of ototoxic reactions to medications, or who might need more support than usual to manage their hearing aids, leading to improved clinical guidelines.
Evidence-based policy-making has not yet fully gained acceptance, as illustrated by a statement from the Organization for Economic Co-operation and Development (2017) that noted, “feeding high-quality evidence into policy-making remains difficult but is essential for improving public interventions. Governing better through evidence-informed policy-making, through big data use, requires building capacity for the effective use and demand of evidence at all levels of government.” However, with time, this will likely change.
It is acknowledged that policy-making will always be challenging, even when there is a strong evidence base for policy actions. This is because, while academic tools for generating evidence and policy-makers’ tools for generating public health policies progress in parallel, they use different languages, values, rewards, and timescales (Choi et al. 2005). Further, political knowledge and engagement will always be required to move evidence into practice, and political agendas will always influence this process (Orton et al. 2011; Andermann et al. 2016). Problems that require a rapid response solution will always arise, funds will always be limited, and policymakers will need the necessary skills in economics, statistics, and relevant-scientific disciplines to interpret the evidence.
BIG DATA IN HEALTHCARE
Big data are relatively easy to collect in the context of healthcare because digitized data are available from many sources, such as electronic health records, pharmaceutical data, test results, clinical trials, sensors, wearables, mobile apps, social media, and behavioral and socioeconomic indicators (Raghupathi & Raghupathi 2014). Indeed, an increasing number of organizations have recognized the potential of using big data in the healthcare context to predict disease susceptibility, select an optimal therapy, improve prognostic models, understand responses to a particular intervention, and improve clinical decision-making (Obermeyer & Emanuel 2016; Polanczyk et al. 2019). Not only can big data be used to facilitate investigations of human behaviors and decision-making processes but it also can reveal patterns and associations that currently remain hidden (Ullah et al. 2017).
There are, of course, pitfalls with using big data to draw conclusions. These include inadvertent bias, breaches of privacy and security, issues around ownership of data, lack of transparency, challenges with integrating data from different systems, and the challenges with differentiating associations versus causal relationships among variables (Shiffrin 2016; Topol 2019; Yaffe 2019). Nonetheless, the application of big data to healthcare is inevitable (Murdoch & Detsky 2013), and indeed most companies that provide healthcare tools and interventions have embraced digitization (McKinsey and Company 2015).
As with other medical disciplines, big data approaches have been used by some in the field of audiology. For example, Mellor et al. (2018) applied big data mining of hearing aid data logging parameters with a view to using the findings to guide and assess hearing aid fitting procedures; Mahmoudi et al. (2018) used healthcare data from US Medicare recipients with self-reported hearing loss to examine the impacts of hearing aid use on hospitalizations and healthcare use, and Willink et al. (2019) conducted a cost-benefit analysis to Medicare of patients with hearing loss who used or did not use hearing care services. In the latter studies, hearing aid/hearing care usage resulted in substantial cost savings.
HEARING HEALTHCARE POLICY-MAKING
Age-related hearing loss is the third leading cause of years lived with disability (Vos et al. 2017), it has an annual estimated cost to Europe alone of between 555 and 675 billion euros depending on hearing aid ownership (Shield 2018), and has been described as one of the modifiable risk factors for dementia (Livingston et al. 2017). However, the population with hearing loss is underserved because few public health policies focus on prevention, intervention, and rehabilitation for age-related hearing loss (Reavis et al. 2016). On a positive note, the World Health Assembly recently ratified unanimously the 2017 Resolution on the Prevention of Deafness and Hearing Loss that highlights the urgent need for evidence to underpin the policy-making process for hearing care (World Health Organization 2017). Among other things, it noted that too few resources are allocated to build an infrastructure that improves financial access to hearing care through private or public healthcare coverage. The lack of public health policies supporting hearing healthcare has been attributed to a lack of evidence supporting policies around, for example, routine hearing screening for adults (Moyer 2012), intervention to promote hearing aid use (Barker et al. 2016), and support for bilateral as opposed to unilateral hearing aid fitting (Schilder et al. 2017). However, research supporting such policies is gradually changing this (Ferguson et al. 2017).
THE EVOTION PROJECT
EVOTION is an ongoing multisite study in which a research prototype of a public health policy decision-making platform is being used to illustrate how big data could provide an evidence base for formulating and evaluating the impacts of public health policy. Retrospective clinical data are being analyzed in combination with data from prospective real-world data collected from multiple sources (see below).
Data collection is ongoing, and in parallel, the concept of a public health policy decision-making platform is being evaluated with potential stakeholders through a series of focus groups. A total of 979 participants have been enrolled in the study from three test sites in the United Kingdom (Guy’s and St Thomas’ NHS Foundation Trust, University College London, James Paget University Hospitals NHS Foundation Trust), two in Greece (Hippokrateion Hospital, University of Athens and Athens Medical Center), and one in Denmark (Eriksholm Research Centre). With the exception of the Danish site, all participants were referred to the study following a routine audiology appointment. Some study participants were first-time users of hearing aids, others had used hearing aids in the past. All received routine clinical care during study participation. Participants at the Eriksholm site were recruited from a research database and were invited to participate based on their meeting eligibility requirements (age 18 years or older with mild to severe sensorineural HL, a Montreal Cognitive Assessment [MoCA] score > 22, being willing and able to use hearing aid for at least 2 hours a day, and being willing and able to use a smartphone).
The study requires three clinic visits, after which real-world data are collected over a period of 1 year. After being identified as a potential participant, individuals were informed about the study and provided with study information. Those willing to participate were scheduled to attend the first study visit at which study eligibility was checked, informed consent was obtained, standard audiological prehearing aid fitting assessments were made, and the Glasgow Hearing Aid Benefit Profile (Gatehouse 1999) was completed. In addition, participants filled out three study questionnaires: MoCA (Nasreddine et al. 2005; Konstantopoulos et al. 2016), the Hospital Anxiety and Depression Scale (Zigmond & Snaith 1983; Michopoulos 2008), and the Health Utility Index Mark 3 (Horsman et al. 2003). At the second study visit, participants were fitted with standard Oticon Opn hearing aids that had been adapted to log information additional to that routinely collected. Specifically, on a minute-by-minute basis, the hearing aids log sound pressure levels, signal to noise ratio, hearing aid use behavior (i.e., changes made to settings), and the hearing aid settings themselves. Participants were fitted with one or two hearing aids as appropriate using the manufacturer’s fitting algorithm, and the hearing aid output was verified with real-ear measurement. Participants were provided with a mobile phone (Samsung Galaxy A3) for running the EVOTION study application (app), and which recorded their physical location. The EVOTION app also allowed participants to self-administer a variety of audiological tests (speech-in-babble, digit recall, pure tone threshold testing at 4 kHz) to make self-ratings of hearing aid benefit and noise exposure and to conduct auditory training. The app was also used for transferring data from the phone to a data repository, as further described later. The third study visit took place 6 to 8 weeks after the hearing aid fitting. At this visit, the hearing aids were fine-tuned as necessary, and participants repeated the MoCA, Glasgow Hearing Aid Benefit Profile, Health Utility Index Mark 3, and Hospital Anxiety and Depression Scale. Following this visit, data were collected automatically for 1 year from the hearing aids, EVOTION app, and mobile phone. On completion of the study, participants are permitted to keep the study hearing aids and the mobile phone. For further details about the study protocol, specific variables collected, and study design, see Dritsakis et al. (2018).
The data logged by the hearing aids are sent via a low energy Bluetooth connection to the mobile phone and encrypted. Once per day, when the mobile phone has enough power and good network connection, the stored encrypted data are transmitted to the EVOTION data repository and locally stored data on the mobile phone are deleted. Data in the repository are stored in an anonymized form.
The data stored in the data repository are being analyzed using big data analytics and modeling techniques. In the future, it is envisaged that the repository would be accessible via a user-friendly interface to a variety of different stakeholders, such as government organizations, healthcare providers, and clinical researchers. Stakeholders would input text-based queries into the repository to answer policy-related questions. Queries could be “bottom-up” or “data-driven” and could ask such questions as “What policies are indicated based on relationships between a number of variables in the dataset?.” Conversely, the stakeholder could submit a “top-down” query. That is, a stakeholder could submit a hypothesis-driven query to answer questions such as “Is a particular policy supported by data?” They would receive feedback on whether a user-specified criterion (e.g., significant effect size of differences between variables) has been reached. It will be possible to implement analytical workflows customized for any particular use case. For example, a significant relationship indicating that hearing aid uptake depends on an interaction between hearing aid subsidization and age from a bottom-up query might motivate an age-dependent hearing aid subsidization policy. For data variables with known causal relationships, based, for instance, on previous literature, stakeholders could also ask “What would the impacts of implementing a particular policy be?” and activate an analytical workflow that will first produce a predictive mixed model of the variables in question and then simulate outcomes of the dependent variable.
Currently, we are conducting data analyses with a preliminary dataset from the EVOTION repository that has been fully synthesized using DataSythesizer (Ping et al. 2017). The dataset was generated with constraints to hide sensitive private information but to retain certain statistical information and relationships between attributes in the original data. This dataset is publicly available through zenodo.org (https://doi.org/10.5281/zenodo.2668210) and has been described in an accompanying open-access paper (Christensen et al. 2019b). To the knowledge of the authors, this is the first case of open-access real-world hearing aid data for research.
To date, we have used this synthetic dataset to develop a model that estimates the risk of noise-induced hearing loss by modeling the combined impacts of measured ambient noise from the hearing aid, estimated sound levels at the eardrum following hearing aid processing, the moderating effects of the hearing loss, and recovery from temporary threshold shift assessed using the app (Dudarewicz et al. 2018a). The model showed that a study population of bartenders were significantly more likely to suffer from >10 dB temporary threshold shifts due to noise exposure than the general population (Dudarewicz et al. 2018b). This type of analysis could be used to expand guidelines aimed at preventing noise-induced hearing loss among other populations.
We have also found evidence that environmental sounds are significantly associated with hearing aid usage patterns (Christensen et al. 2019a). This was done by applying a generalized linear mixed model to predict the hourly hearing aid usage (in minutes per hour) from the momentary sound environment encountered by the users. Here, the sound environment sampled every minute by the users’ hearing aids (five acoustic features, each in four frequency bands) were first quantified by three independent factors: sound pressure levels, sound clarity (i.e., the ratio between signal and noise), and sound variability within each hour of the day. These three factors were then used as predictors to model hourly hearing aid usage. As seen in Table 1, the data showed that greater hearing aid usage was significantly associated with higher sound and noise levels and more diverse sound environments. Because the predictor variables were significantly associated with hourly hearing aid usage, the model can be used to simulate a distribution of expected hearing aid usage in the population given changes to the sound environment. Figure 1 shows this in the form of a density histogram showing the observed, predicted, and simulated daily hearing aid use for the dataset. It illustrates that the predicted data are an accurate representation of the observed data and that the simulated data not merely represents a shift in mean hearing aid usage but also a change in the form of the distribution. A policymaker could use this information to simulate hearing aid uptake and usage if urban planning organizations were to project an increase in everyday acoustic noise due to changed requirements for official noise prevention initiatives. Note that, to validate the accuracy of such a simulation, the predictive performance of the model would need to be tested on data from subpopulations of a data repository that differ in their environmental sound exposure profiles.
TABLE 1.
Mixed-Model Coefficient Values With 95% Confidence Intervals in Parentheses for Predicting Hourly Hearing Aid Usage by Factors of the Sound Environment
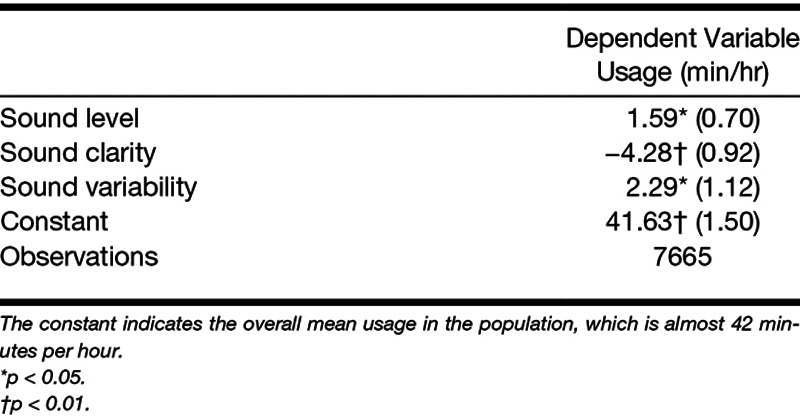
Fig. 1.
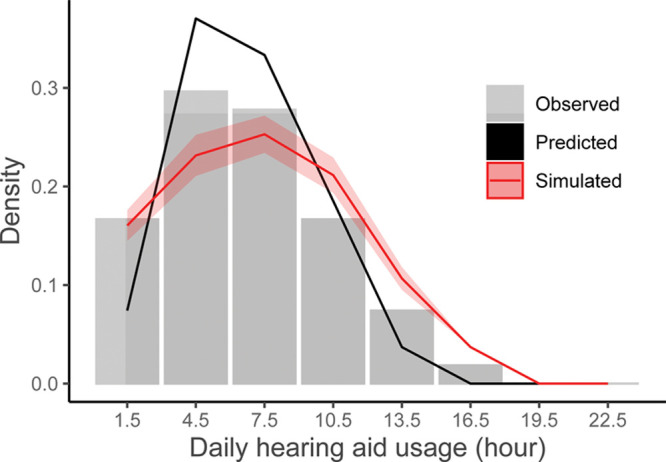
Density histogram of observed daily hearing aid usage (gray bars) together with the model prediction (solid black line) and a simulation of usage times (solid red line with confidence intervals) given changes to the sound environment. The x axis represents daily hearing aid usage intervals (bin-centers). The width of each bin is 3 hr. The y axis represents the density, that is, how likely the different usage intervals are to occur. For the observed data, the density for a specific usage time is equal to the proportion of observed usage times within that interval, divided by the total number of usage times. The prediction is based on a linear mixed model of the observed hearing aid usage per hour predicted by acoustic parameters sampled by the hearing aids (see model coefficients in Table 1). The simulation was produced by increasing the observed levels by 20 dB SPL and decreasing the sound clarity by decreasing the signal to noise ratio by 5 dB SPL to mimic a scenario that worsens the sound environment for hearing aid users.
Finally, we have examined the association between users’ absolute hearing aid usage, their physical activity levels and the sound environments they encounter. In Figure 2, we show the relationship between activity levels and daily average hearing aid usage times. Activity levels were derived using the GPS data to compute daily distance moved and dividing it with the daily hearing aid usage time in minutes, yielding activity defined as an average daily speed in units of meters per minute. The figure shows that higher activity levels are associated with more daily hearing aid use. While we cannot attribute a causal effect to these findings, such analyses can provide insight into the effect that hearing aid wear has on general life activities or vice versa and, thus, establish evidence concerning potential improvements in metrics with real-life relevance, such as quality of life scales.
Fig. 2.

Grand mean daily activity (across days) by individual is plotted against their grand mean daily hearing aid usage (x axis). The size of each dot indicates the relative standard error. The significant correlation indicates that individuals with a more active life while wearing hearing aids also exhibit higher hearing aid usage and/or vice versa.
A major advantage of a platform like EVOTION is the possibility of ongoing data collection which would mean that models could be continuously updated with every query. Not only could such a platform adapt to changing technologies and demographics but it could also document the impacts of policy, environmental, and societal changes over time, thus providing evidence to infer a causal association. This would also enable policymakers to assess whether a policy that has been implemented meets its objectives, whether the policy has had unintended consequences and change recommendations accordingly—this is typically a challenging step in policy-making (Basu et al. 2017).
CAVEATS, CAUTIONS, AND FUTURE STUDY
There are several caveats that must be considered when using big data. First, intervention-type simulations, such as, how much does Y change if X changes by this amount, require statistical methods that consider the causal effects among variables (Shardell & Ferrucci 2018). Care must be taken not to attribute causality when the association might be correlational. For example, the association we have found between the activity level and hearing aid usage could arise because hearing aid use leads people with hearing loss to be more active, or that when being active, people use their hearing aids more, or both could be confounded by a tertiary variable such as general health, that is, healthy people are active and wear their hearing aids.
Second, data collected and stored in any data repository like EVOTION are highly sensitive. If data are collected from hearing aids, the purchaser must be made aware of how the data are stored and collected and must be given the opportunity to opt-out of sharing their data. The opportunity to opt-out of data sharing can of course lead to bias in the data available for analysis. This necessitates some form of consent procedure. In addition, to conserve data privacy in accordance with General Data Protection Regulation, access to the data could only take place via a dedicated web interface that does not allow the extraction of any raw data points. Data privacy will always be a major concern and must impact how and by whom personal data in a data repository are accessed.
Finally, maintaining and accessing a platform like EVOTION will require technical, administrative, and financial support. Many questions about this remain. For example, how will the platform be maintained and by whom? Who will enter and own the data? How will stakeholders gain access to the platform? Who should those stakeholders be? With which manufacturers’ technology will it be compatible? And very importantly, how can we minimize sample bias when a data collection platform requires high-end hearing aids and use of a smartphone? Nonetheless, we believe our research prototype provides strong support that such a platform has huge potential value for policymakers, clinicians, and patients alike.
SUMMARY
Use of big data in healthcare and policy-making is still in its infancy, in part due to high costs, limited interoperability of information technologies, and privacy and security challenges. But all indications suggest that with time, big data will play a prominent role in healthcare and policy-making. We hope that our research prototype EVOTION platform illustrates the value of using big data for policy-making by providing high-quality evidence that could be used to formulate and evaluate the impact of hearing healthcare policies.
ACKNOWLEDGMENTS
This project has received funding from the European Commission’s Horizon 2020 research and innovation program under grant agreement no. 727521.
Footnotes
G.H.S. took the lead in writing the article and contributed to the interpretation of the results. J.H.C. conceived and performed the data analyses, wrote sections of the article, and contributed to the interpretation of the results. J.G. provided critical feedback on the article. N.H.P. conceived and planned the experiments and contributed to the interpretation of the results. A.S., G.S., and D.-E.B. conceived and planned the experiments. All authors provided critical feedback and helped shape the research, analysis, and article.
To the best of our knowledgethe authors have no conflict of interest.
REFERENCES
- Andermann A., Pang T., Newton J. N., et al. Evidence for Health II: Overcoming barriers to using evidence in policy and practice. Health Res Policy Syst, 2016). 14, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker F., Mackenzie E., Elliott L., et al. Interventions to improve hearing aid use in adult auditory rehabilitation. Cochrane Database Syst Rev, 2016). 8:CD010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Meghani A., Siddiqi A. Evaluating the health impact of large-scale public policy changes: Classical and novel approaches. Annu Rev Public Health, 2017). 38, 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke K., Benke G. Artificial intelligence and big data in public health. Int J Environ Res Public Health, 2018). 15, pii: E2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B. C., Pang T., Lin V., et al. Can scientists and policy makers work together? J Epidemiol Community Health, 2005). 59, 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. H., Pontoppidan N. H., Anisetti M., et al. Improving hearing healthcare with Big Data analytics of real-time hearing aid data. In 2019 IEEE World Congress on Services (SERVICES) (Vol. 2019a). 2642, pp. IEEE; 307–313). [Google Scholar]
- Christensen J. H., Pontoppidan N. H., Rossing R., et al. Fully synthetic longitudinal real-world data from hearing aid wearers for public health policy modeling. Front Neurosci, 2019b). 13, 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner M. S., Lietman T. M., McLeod S. D., et al. Surveillance tools emerging from search engines and social media data for determining eye disease patterns. JAMA Ophthalmol, 2016). 134, 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dritsakis G., Kikidis D., Koloutsou N., et al. Clinical validation of a public health policy-making platform for hearing loss (EVOTION): Protocol for a big data study. BMJ Open, 2018). 8, e020978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudarewicz A., Pawlaczyk-Łuszczyńska M., Śliwińska-Kowalska M., et al. Predicting impact of loud incidents on individual hearing for public health policy in the framework of EVOTION. 2018a). Heraclion, Greece: Retrieved September 10, 2019. from http://www.euronoise2018.eu/docs/papers/65_Euronoise2018.pdf. [Google Scholar]
- Dudarewicz A., Zaborowski K., Wolniakowska A., et al. The risk of temporary hearing threshold shift in bartenders. 2018b). 258, No. INTER-NOISE and NOISE-CON Congress and Conference Proceedings (Vol. 2, pp. Institute of Noise Control Engineering; 5041–5052). [Google Scholar]
- Ferguson M. A., Kitterick P. T., Chong L. Y., et al. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev, 2017). 9, CD012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse S. Glasgow Hearing Aid Benefit Profile: Derivation and validation of a client-centered outcome measure for hearing aid services. J Am Acad Audiol, 1999). 10:80–103. [Google Scholar]
- Greer S. L., Bekker M., de Leeuw E., et al. Policy, politics and public health. Eur J Public Health, 2017). 27(suppl_4), 40–43. [DOI] [PubMed] [Google Scholar]
- Gutenberg J., Katrakazas P., Trenkova L., et al. Big data for sound policies: Toward evidence-informed hearing health policies. Am J Audiol, 2018). 27(3S), 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez I., Zhang Y. Using predictive analytics and big data to optimize pharmaceutical outcomes. Am J Health Syst Pharm, 2017). 74, 1494–1500. [DOI] [PubMed] [Google Scholar]
- Horsman J., Furlong W., Feeny D., et al. The Health Utilities Index (HUI): Concepts, measurement properties and applications. Health Qual Life Outcomes, 2003). 1, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. A., Tu J. V., Moineddin R., et al. Interaction between neighborhood walkability and traffic-related air pollution on hypertension and diabetes: The CANHEART cohort. Environ Int, 2019). 132, 104799. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (The Future of Public Health. 1988). Washington, DC: National Academies Press (US). [Google Scholar]
- Katrakazas P., Trenkova L., Milas J., et al. The EVOTION Decision Support System: Utilizing it for public health policy-making in hearing loss. Stud Health Technol Inform, 2017). 238, 88–91. [PubMed] [Google Scholar]
- Kehler D. S. Age-related disease burden as a measure of population ageing. Lancet Public Health, 2019). 4, e123–e124. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos K., Vogazianos P., Doskas T. Normative data of the Montreal Cognitive Assessment in the Greek Population and Parkinsonian Dementia. Arch Clin Neuropsychol, 2016). 31, 246–253. [DOI] [PubMed] [Google Scholar]
- Larsen M., Gulis G., Pedersen K. M. Use of evidence in local public health work in Denmark. Int J Public Health, 2012). 57, 477–483. [DOI] [PubMed] [Google Scholar]
- Lee R. The demographic transition: Three centuries of fundamental change. J Econ Perspect, 2003). 17:167–190. [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., et al. Dementia prevention, intervention, and care. Lancet, 2017). 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- Luo J., Wu M., Gopukumar D., et al. Big data application in biomedical research and health care: A literature review. Biomed Inform Insights, 2016). 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi E., Zazove P., Meade M., et al. Association between hearing aid use and health care use and cost among older adults with hearing loss. JAMA Otolaryngol Head Neck Surg, 2018). 144, 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey and Company. (The role of big data in medicine. 2015). Retrieved from April 10, 2019, from https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/the-role-of-big-data-in-medicine.
- Mellor J., Stone M. A., Keane J. Application of data mining to a large hearing-aid manufacturer’s dataset to identify possible benefits for clinicians, manufacturers, and users. Trends Hear, 2018). 22, 2331216518773632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos I., Douzenis A., Kalkavoura C., et al. Hospital Anxiety and Depression Scale (HADS): Validation in a Greek general hospital sample. Ann Gen Psychiatry, 2008). 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney S. J., Pejaver V. Big data in public health: Terminology, machine learning, and privacy. Annu Rev Public Health, 2018). 39, 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V. A.; U.S. Preventive Services Task Force. (Screening for hearing loss in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med, 2012). 157, 655–661. [DOI] [PubMed] [Google Scholar]
- Murdoch T. B., Detsky A. S. The inevitable application of big data to health care. JAMA, 2013). 309, 1351–1352. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 2005). 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Obermeyer Z., Emanuel E. J. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med, 2016). 375, 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K., Innvar S., Lorenc T., et al. A systematic review of barriers to and facilitators of the use of evidence by policymakers. BMC Health Serv Res, 2014). 14, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development. (Governing better through evidence-informed policy making. 2017). Retrieved April 10, 2019, from http://www.oecd.org/gov/governing-better-through-evidence-informed-policy-making.htm.
- Orton L., Lloyd-Williams F., Taylor-Robinson D., et al. The use of research evidence in public health decision making processes: Systematic review. PLoS One, 2011). 6, e21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping H., Stoyanovich J., Howe B. DataSynthesizer: Privacy-preserving synthetic datasets. 2017). Proceedings 29th International Conference on Scientific and Statistical Database Management (p. 42). [Google Scholar]
- Polanczyk C. A., Ruschel K. B., Castilho F. M., et al. Quality measures in heart failure: The past, the present, and the future. Curr Heart Fail Rep, 2019). 16, 1–6. [DOI] [PubMed] [Google Scholar]
- Pontoppidan N. H., Li X., Bramsløw L., et al. Santurette S., Dau T., Dalsgaard J. C.-L., et al. Data-driven hearing care with time stamped data-logging. 2017). 2017Proceedings of the International Symposium on Auditory and Audiological Research (Proc. ISAAR), Vol. 6: Adaptive Processes in Hearing, August 2017, Nyborg, Denmark: The Danavox Jubilee Foundation, [Google Scholar]
- Prasinos M., Spanoudakis G, Koutsouris D. Towards a Model-Driven Platform for Evidence based Public Health Policy Making. 2017). SEKE 2017 Proceedings of the 29th International Conference on Software Engineering & Knowledge Engineering (pp. Pittsburgh: KSI Research Inc. and Knowledge Systems Institute; 566–571). [Google Scholar]
- Raghupathi W., Raghupathi V. Big data analytics in healthcare: Promise and potential. In Health Inf Sci Syst, 2014). 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavis K. M., Tremblay K. L., Saunders G. How can public health approaches and perspectives advance hearing health care? Ear Hear, 2016). 37, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathé M., Khandelwal S. Assessing vaccination sentiments with online social media: Implications for infectious disease dynamics and control. PLoS Comput Biol, 2011). 7, e1002199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder A. G., Chong L. Y., Ftouh S., et al. Bilateral versus unilateral hearing aids for bilateral hearing impairment in adults. Cochrane Database Syst Rev, 2017). 12, CD012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield B. Evaluation of social and economic costs of hearing impairment 2018. 2018). A report for Hear-It AISBL. Retrieved April 10, 2019, from https://www.hear-it.org/sites/default/files/BS%20-%20report%20files/HearitReportHearingLossNumbersandCosts.pdf.
- Shiffrin R. M. Drawing causal inference from big data. Proc Natl Acad Sci U S A, 2016). 113, 7308–7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardell M., Ferrucci L. Joint mixed-effects models for causal inference with longitudinal data. Stat Med, 2018). 37, 829–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanoudakis G., Kikidis D., Bibas A., et al. Public health policy for management of hearing impairments based on big data analytics: EVOTION at Genesis. 2018). 2017 IEEE 17th International Conference on Bioinformatics and Bioengineering (BIBE) (pp. 525–530). [Google Scholar]
- Topol E. J. High-performance medicine: The convergence of human and artificial intelligence. Nat Med, 2019). 25, 44–56. [DOI] [PubMed] [Google Scholar]
- Tu J. V., Chu A., Donovan L. R., et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): Using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes, 2015). 8, 204–212. [DOI] [PubMed] [Google Scholar]
- Ullah F., Habib M. A., Farhan M., et al. Semantic interoperability for big-data in heterogeneous IoT infrastructure for healthcare. Sustain Cities Soc, 2017). 34:90–96. [Google Scholar]
- van de Goor I., Hämäläinen R. M., Syed A., et al. Determinants of evidence use in public health policy making: Results from a study across six EU countries. Health Policy, 2017). 121:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Abajobir A. A., Abate K. H., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet, 2017). 390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willink A., Reed N. S., Lin F. R. Cost-benefit analysis of hearing care services: What is it worth to medicare? J Am Geriatr Soc, 2019). 67, 784–789. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (Prevention of deafness and hearing loss. Seventieth World health assembly WHA70.13 Agenda item 15.8 31 May 2017. 2017). Retrieved April 10, 2019, from http://apps.who.int/gb/ebwha/pdf_files/wha70/a70_r13-en.pdf.
- World Health Organization. (2019). Health topics: Health policy. Retrieved April 10, 2019, from http://www.who.int/topics/health_policy/en/.
- Yaffe M. J. Emergence of “big data” and its potential and current limitations in medical imaging. Semin Nucl Med, 2019). 49, 94–104. [DOI] [PubMed] [Google Scholar]
- Young S. D., Rivers C., Lewis B. Methods of using real-time social media technologies for detection and remote monitoring of HIV outcomes. Prev Med, 2014). 63, 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A. S., Snaith R. P. The hospital anxiety and depression scale. Acta Psychiatr Scand, 1983). 67, 361–370. [DOI] [PubMed] [Google Scholar]


