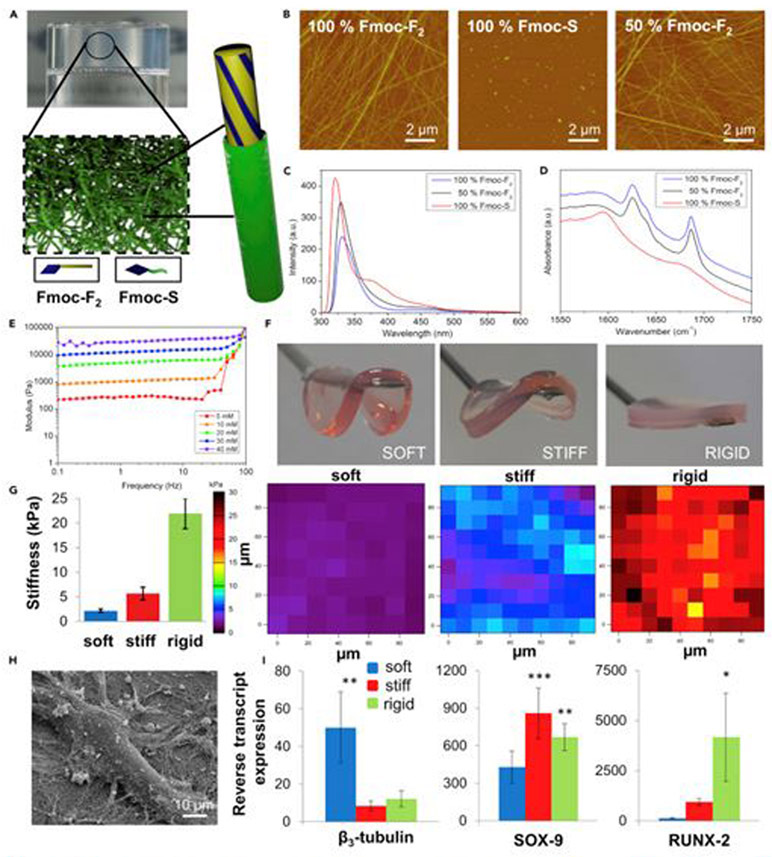
Figure 16.
Neuronal, chondrogenic, and osteogenic differentiation based on rigidity of hydrogel stiffness. (A) Schematic of proposed core-shell nanostructures. (B) AFM images of Fmoc-F2, Fmoc-S, and the 1:1 mixture of the two. (C) Fluorescence emission spectra corresponding to monomeric emission of the fluorenyl moiety (sharp peak at 320 nm), formation of aggregated excimers (broad peak at 460-480 nm), and differential fluorenyl organization upon formation of spherical aggregates (peak at 380 nm). (D) FITR spectra demonstrating that Fmoc-F2 possesses peptide amide I modes consistent with well-ordered β-sheet-like arrangement (band at 1,625 cm−1) and ordered carbamate moiety (band at 1,687 cm−1). For Fmoc-S, carboxylate peak evident at 1,590 cm−1 loses intensity and broadens in the mixture of the two components, indicating co-assembly. (E) Oscillatory rheology of the gels demonstrates elastic moduli of different concentration in gels. Stiffness increases with concentration. (F) Macroscopic images for 10, 30, and 40 mM gels. (G) Young’s modulus and AFM stiffness maps of soft, stiff, and rigid gels. (H) SEM image of single MSC attached to Fmoc-F2/S gel fibers. (I) Assessment of pericytes for the expression of β3-tubulin (neural cells), SOX-9 (chondrocytes), and RUNX2 (osteoblasts) on each hydrogel type. Primary expression of β3-tubulin was observed on the 1 kPa (soft) surface, SOX-9 was observed on the 13 kPa (stiff) surface, and RUNX2 was observed on the 32 kPa (rigid) surface after 1 week of culture. Cells were only cultured in basal media with no differentiation-enhancing factors, and so differentiation is purely substrate driven. Statistics were obtained by ANOVA and Bonferroni post hoc tests; the control was pericytes on glass coverslips with standard media, *p < 0.05, **p < 0.01, and ***p < 0.001. Reproduced with permission from Alakpa et al.252 Copyright 2016, Elsevier.