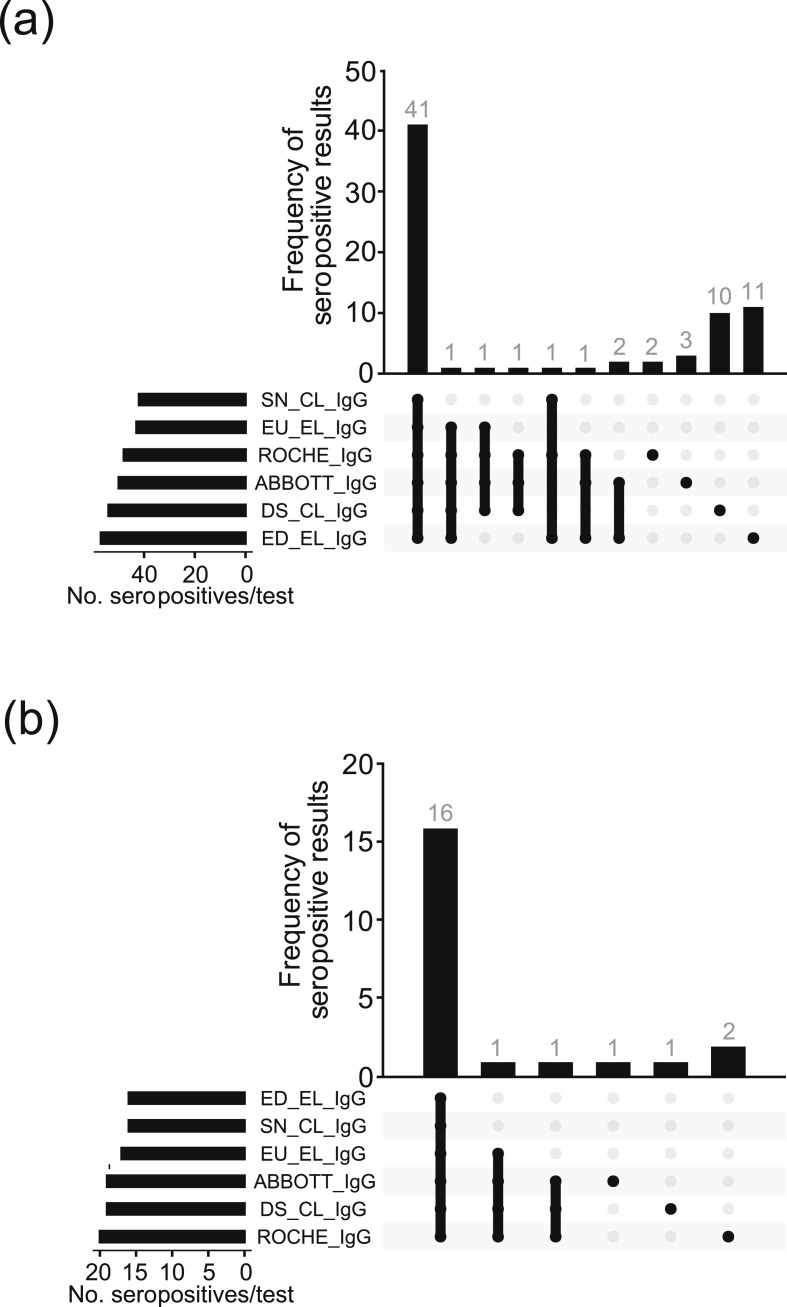
Fig. 2.
A comparison of test performance between the six serological IgG assays in 600 participants for whom all six assays were performed. (a) Upset plot of all antibody-positive participants and (b) Upset plot of previously SARS-CoV-2 PCR-positive participants. Abbreviations: SN.2019-nCoV IgG kit (Snibe Co., Ltd., Shenzhen, China); EU..SARS-COV-2 IgG ELISA kit (Euroimmun, Lübeck, Germany); DS..SARS-CoV-2 S1/S2 IgG CLIA kit (DiaSorin, Saluggia, Italy); ED..EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics Inc., San Diego, USA).