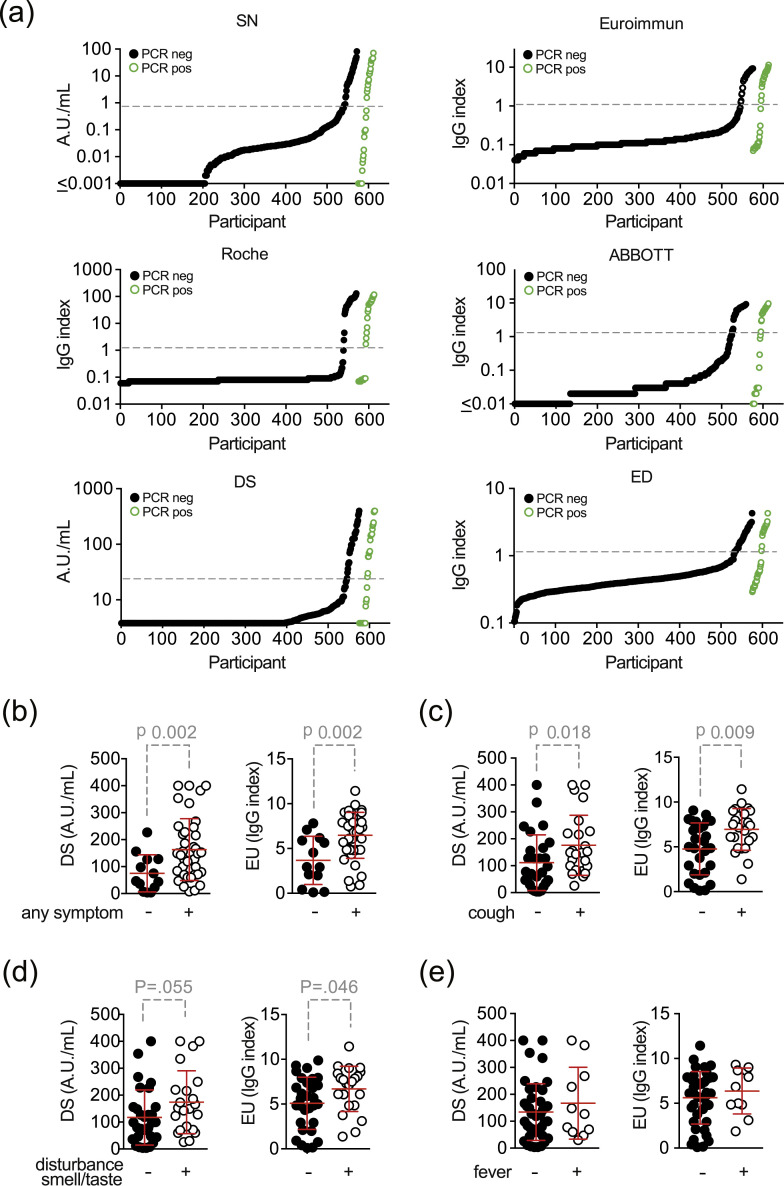
Fig. 4.
Semi-quantitative test results of (a) all six assays from all participants with serology shown for previously PCR-positive (black dots) and PCR-negative (green circles) participants. Dashed grey-line indicates threshold for positive test results according to the manufacturer. (b–e) Semi-quantitative test results of participants with self-reported symptoms (with two-sided p values of the Wilcoxon–Mann–Whitney test): (b) any symptom, (c) cough, (d) taste/smell disorder and (e) fever. When results were below or above the detection limit of the assay, values were set to the respective lower or upper boundaries. Abbreviations: SN..2019-nCoV IgG kit (Snibe Co., Ltd., Shenzhen, China); EU..SARS-COV-2 IgG ELISA kit (Euroimmun, Lübeck, Germany); DS..SARS-CoV-2 S1/S2 IgG CLIA kit (DiaSorin, Saluggia, Italy); ED..EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics Inc., San Diego, USA).