Abstract
Background and aims
Recent studies show that obesity is a risk factor for hospital admission and for critical care need in patients with coronavirus disease 2019 (COVID-19). The aim was to determine whether obesity is a risk factor for unfavourable health outcomes in patients affected by COVID-19 admitted to ICU.
Methods and results
95 consecutive patients with COVID-19 (78 males and 18 females) were admitted to ICU and included in the study. Height, weight, BMI, Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, CRP, CPK, ICU and hospital length of stay and comorbidities were evaluated. Participants with obesity had a lower 28 day survival rate from ICU admission than normal weight subjects. Cox proportional hazard model-derived estimates, adjusted for age, gender and comorbidity, confirmed the results of the survival analysis (HR:5.30,95%C.I.1.26–22.34). Obese subjects showed longer hospital and ICU stay as compared with normal weight counterpart.Subjects with obesity showed significantly higher CRP and CPK levels than normal weight subjects.
Conclusion
In individuals with obesity, careful management and prompt intervention in case of suspected SARS-CoV-2 infection is necessary to prevent the progression of the disease towards severe outcomes and the increase of hospital treatment costs.
Keywords: SARS-CoV-2, COVID-19, Obesity, Body mass index, Intensive care unit
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 infection, includes a spectrum of illness; from asymptomatic infection [1] to severe pneumonia characterized by acute respiratory injury in about 20% of patients presented for medical care [2]. The risk factors associated with disease severity included increased age, diabetes, immune suppression and organ failure [2].
Preliminary studies in China, where SARS-CoV-2 initially spread, showed that obesity, especially in men, significantly increased the risk of developing severe pneumonia in infected patients.
Among 383 COVID-19 patients from Shenzhen, overweight was associated with an 86% higher, and obesity with a 142% higher, risk of developing severe pneumonia than patients of normal weight, when using statistical models that controlled for potential confounders [3].
Recent studies showed that obesity is a risk factor for hospital admission and critical care need [4,5].
In particular Petrilli et al. [4] showed a significantly strong association between older age, obesity, heart failure and chronic kidney disease with hospitalization risk, with much less influence of race, smoking status, chronic pulmonary disease and other forms of heart disease. Moreover they found that the chronic condition with the strongest association with critical illness was obesity, with a odds ratio of 4.3 for BMI between 30 and 40/m2, and of 6.3 for BMI >40 kg/m2, even higher than any cardiovascular or pulmonary disease.
Similar results were obtained by Lighter et al. in a population of patients aged <60 years, generally considered a lower risk group for COVID-19 disease severity, confirming that obesity appears to be a previously unrecognized risk factor for hospital admission and critical care need [5].
This has important and practical implications, as nearly 40% of US adults and 21% of European ones are obese with a BMI >30 [[6], [7], [8]]. Obesity in people <60 years of age is a newly identified epidemiologic risk factor which may contribute to the increased morbidity rates that are experienced in the US [6].
Recent wide population studies from China [2] and from the Lombardy region of Italy [9] that have reported comorbidities in COVID-19 patients unfortunately did not provide any data on body weight and height, which are used to estimate adipose tissue mass by calculating the BMI.
A descriptive study of a small sample of 24 (63% were men) critically ill patients diagnosed with COVID-19 in the Seattle region was among the first to report BMI data (3 patients with a BMI in the normal category, 7 with overweight, 13 with obesity and 1 with missing data). Although the numbers are too small for meaningful statistical analyses, 85% of the patients with obesity required mechanical ventilation and 62% of the patients with obesity died. These proportions are greater than those in patients without obesity, in which 64% required mechanical ventilation and 36% died [10]. These results are in line with Simonnet et al. that observed an increased need for invasive mechanical ventilation in subjects with BMI≥ 35 kg/m2 and Williamson reporting increased death risk in subjects in 17 million adults from the NHS [11,12].
Obesity is a risk factor for hospital admission and for critical care need [13], but only a few studies have investigated intensive care unit (ICU) main outcomes [10,12,14].
The primary aim of the present study was to evaluate if obesity is a risk factor for unfavourable health outcomes in critically ill COVID-19 patients to our ICUs.
The secondary aim was to compare, in different BMI groups, the observed trend of CRP and CPK, as potential clues of the pathophysiological mechanisms linking disease severity with BMI.
Methods
The study population consisted of 95 COVID-19 patients from the REINSURE-ARDS registry (62.46 ± 11.81 years; with mean BMI 29.20 ± 3.49 kg/m2, 17 women), consecutively admitted to the ICU of the University Hospital Integrated Trust of Verona between March 8th and March 30th, 2020. All subjects had microbiologic confirmation of COVID-19 diagnosis by sampling of oro/nasopharyngeal swab. Data was recorded by the attending physician team reviewing medical charts, radiologic and laboratory records. Upper respiratory specimens were obtained in accordance with the WHO indications [15], while venous blood sampling was drawn in agreement with the current guidelines [16].
This study was approved by the ethical board of the University of Verona (Prog 1946CESC, Prot 72485 12/11/2018). Patient identification remained anonymous and written informed consent was obtained for participants. All tests and procedures were performed in accordance with the internal standards of care.
The ICU admission criteria and treatment decisions, including the determination of the need for intubation and type of administered antibiotic and antiviral therapy, were not standardized and were made by the attending medical team.
To determine the severity of illness upon ICU admission, the Acute Physiology And Chronic Health Evaluation (APACHE) II score [17] was determined in all patients within 24 h of ICU admission. In addition, organ failure was assessed using the Sequential Organ Failure Assessment (SOFA) scoring system [18].
Anthropometric measures
Patients’ height and weight were recorded at the beginning of hospitalization as previously reported [19]. Body mass index (BMI) was calculated as the ratio between weight and height squared (kg/m2). The study population was divided on the basis of BMI in normal weight subjects (BMI<27 kg/m2), overweight (BMI between 27 and 29.9 kg/m2) and subjects with obesity (BMI≥30).
Biochemical measures
Repeated venous blood samples for C-reactive protein (CRP) and creatin phosphokinase (CPK) levels were obtained after overnight fasting.
10.1016/j.numecd.2020.12.009For the measurement of CRP a kit was used for the quantitative determination of CRP in serum (CRP ROCHE applied on ROCHE/HITACHI COBAS 702, Roche Diagnostics GmbH, Mannheim, Germany). Measurement uncertainty declared 5.8%. The coefficient of variation (CV) of annual CQI to 6 mg/L is 6%.
Creatine phosphokinase (CPK) concentration was determined on a fully automated analyser ANALIZER ROCHE/HITACHI COBAS 702 (Roche Diagnostics GmbH, Mannheim, Germany). A multicenter evaluation of the within-run precision of the Advia 2120 system showed CV always lower than 0.7% for CPK.
Procalcitonin concentration were determined on the fully automated analyser ANALIZER ROCHE/HITACHI COBAS 801 (Roche Diagnostics GmbH, Mannheim, Germany). A multicenter evaluation of the within-run precision of the ROCHE/HITACHI COBAS 801 system showed CV always lower than 1.8% for PCT.
D-dimer concentration were determined on the fully automated analyser ANALIZER ACL TOP 700 (Roche Diagnostics GmbH, Mannheim, Germany). A multicenter evaluation of the within-run precision of the Advia 2120 system showed CV of 2% for D-dimer.
Further routine variables, such as sodium, potassium, creatinine and white blood cells where also collected at ICU admission and reported.
Study outcomes
Our primary outcome (goal) was to assess if obesity is associated with mortality, ICU length of stay (LOS) and hospital LOS. Our secondary outcome (goal) was to assess if obesity condition can influence the trend of inflammation as evaluated with CRP and critically ill induced myopathy as evaluated with CPK.
Statistical analysis
Results are shown as mean ± SD. Variables not normally distributed were log-transformed before analysis. Discrete variables are expressed as counts (percentage) and continuous variables as means ± standard deviation (SD). For the demographic and clinical characteristics of the patients, differences between groups were assessed using the chi-squared test and Fisher's exact test for categorical variables and the Student's t-test or Mann–Whitney U test for continuous variables. Differences in mortality rates across the three groups of participants were preliminarily evaluated fitting Kaplan–Meier survival curves adjusted for age. Cox proportional hazard models and logistic regression models were used to assess the risk of death.
Hazard Ratio (HR) and 95% Confidence intervals (95%C.I.) were estimated to investigate the association of the three study groups with the risk of mortality. Three models were fitted for each outcome: unadjusted, age and gender adjusted and adjusted for all potential confounders. Candidate variables to be included in the third model were selected on the basis of the biological and clinical plausibility as risk factors for mortality (age, sex, smoking status, coronary heart disease, congestive heart failure, hypertension, diabetes, neurological pathology, chronic obstructive pulmonary disease, chronic renal failure, immunodepression and cancer). Additional models such as interaction terms BMI x age and BMI x sex were also included.
Students t-test for impaired data was used to compare the trend of different biochemical variables between normal weight subjects, overweight and subjects with obesity.
The BMI value associated with higher mortality was evaluated by means of the receiver operating characteristics (ROC) curve, and was obtained by plotting estimates of the true positive rate (i.e. sensitivity) against the false positive rate (1-specificity) for each possible BMI value. Youden's index was then calculated.
A p-value of 0.05 or less was considered to be statistically significant.
Data analysis was conducted using SPSS 22.0 software (Chicago, IL, USA) [20].
Results
Table 1 shows the main characteristics of the study population (mean ± SD) at baseline, divided in normal weight, overweight and subjects with obesity. 95 subjects, 78 men and 17 women (17.9%) were included in the study.
Table 1.
Baseline demographic, anthropometric and clinical characteristics in different BMI groups (females, n = 78; males, n = 18).
| Normal weight (n = 28) |
Overweight (n = 33) |
Obese (n = 34) |
P | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Min-Max | Mean ± SD | Min-Max | Mean ± SD | Min-Max | ||
| Age (years) | 63.18 ± 12.86 | (29–80) | 61.97 ± 12.77 | (33–82) | 62.35 ± 10.16 | (37–86) | NS |
| Sex (male) n (%) | 24 (86%) | 29 (88%) | 25 (74%) | NS | |||
| Height (m) | 1.74 ± 0.09 | (1,62–1.96) | 1.72 ± 0.08 | (1.4–1.86) | 1.71 ± 0.07 | (1.58–1.84) | NS |
| Weight (Kg) | 76.48 ± 9.69 | (56–92) | 86.29 ± 8.28a | (57–103) | 95.79 ± 8.71a | (77–112) | <0.001 |
| BMI (Kg/m2) | 25.21 ± 1.66 | (20.82–26.99) | 28.87 ± 0.79a | (27.16–29.98) | 32.81 ± 2.20a | (30.08–40.46) | <0.001 |
| CRP (mg/dL) | 150.29 ± 111.18 | (19–410) | 122.73 ± 71.96 | (30–290) | 146.88 ± 75.23 | (6–298) | NS |
| PCT (ng/mL) | 6.94 ± 26.91 | (0.06–143) | 0.72 ± 1.02 | (0.03–5.14) | 0.58 ± 1.07 | (0.05–6.27) | NS |
| D-Dimer (ng/mL) | 2484.69 ± 3147.00 | (120–10,000) | 1507.47 ± 1525.41 | (0–6773) | 1595.67 ± 1487.38 | (330–6999) | NS |
| CPK (U/L) | 158.59 ± 201.40 | (0–840) | 282 ± 254.76a | (27–954) | 310.39 ± 389.18 | (24–1750) | NS |
| Sodium (mmol/L) | 137.36 ± 2.97 | (132–145) | 137.18 ± 5.71 | (128–152) | 136.41 ± 4.50 | (121–144) | NS |
| Potassium (mmol/L) | 3.82 ± 0.48 | (2.92–5.23) | 3.80 ± 0.42 | (3.08–4.8) | 3.85 ± 0.43 | (3.08–4.84) | NS |
| Creatinine (mg/dL) | 1.18 ± 1.02 | (0.56–5.67) | 0.97 ± 0.35 | (0.47–1.93) | 0.87 ± 0.34 | (0–1.91) | NS |
| WBC (10ˆ6/mL) | 9.01 ± 4.36 | (3.44–21.03) | 8.47 ± 3.16 | (2.98–14.53) | 8.51 ± 3.78 | (2.4–21.71) | NS |
| SOFA preIOT | 5.82 ± 2.52 | (3–11) | 5.7 ± 2.11 | (3–10) | 6.26 ± 2.54 | (3–14) | NS |
| APACHE II | 22.54 ± 9.70 | (7–47) | 24.09 ± 11.10 | (8–44) | 23.64 ± 9.20 | (8–53) | NS |
| LOS ICU (days) | 14.46 ± 9.47 | (1–32) | 13.58 ± 8.47 | (3–40) | 18.29 ± 12.31 | (1–40) | NS |
| LOS Hospital (days) | 23.61 ± 10.05 | (2–40) | 22.88 ± 9.70 | (4–40) | 26.59 ± 12.7 | (4–43) | NS |
| Hypertension | 10 (35.71%) | 17 (51.51%) | 17 (0.5) | NS | |||
| Hearth Failure | 2 (7.14%) | 1 (3.03%) | 1 (2.94%) | NS | |||
| IHD | 1 (3.57%) | 0 (0%) | 0 (0%) | NS | |||
| Neurological disease | 0 (0%) | 0 (0%) | 1 (2.94%) | NS | |||
| Type 2 diabetes | 2 (7.14%) | 3 (9.09%) | 13 (38.24%)a | 0.002 | |||
| Dyslipidemia | 0 (0%) | 1 (3.03%) | 1 (2.94%) | NS | |||
| Immunodepression | 8 (28.57%) | 3 (9.09%) | 8 (23.53%) | NS | |||
| CKD | 5 (17.86%) | 1 (3.03%) | 9 (26.47%) | 0.045 | |||
| Cancer | 1 (3.57%) | 1 (3.03%) | 1 (2.94%) | NS | |||
p < 0.05.
All patients were COVID-19 positive as confirmed by Nasopharyngeal-swab specimens which were performed before or within the first 6 h of ICU stay.
As regards therapy all the patients received hydroxychloroquine. Eighty-seven patients (92%) received lopinavir/ritonavir, while 8 patients (8%) were treated with remdesivir. Tocilizumab was administered to 20 patients (21%).
Mean delay between the onset of symptoms and hospital admission was 3.87 ± 2.2 days and between hospital and ICU admission was 2.15 ± 2.37 days.
The mean APACHE II score was 23.47 ± 9.96 and the mean SOFA score pre-intubation was 5.94 ± 2.38. 91 out of 95 subjects required invasive mechanical ventilation. The most common comorbidities were hypertension (47.4.%), followed by heart disease (38.9%), obesity (35.78%) and diabetes (18.95%).
Eighteen patients (18.9%) died during the first 28 days since ICU admission. The mean CPK level adjusted for weight at admission and on day 2 was significantly higher in subjects who died than in survivors.
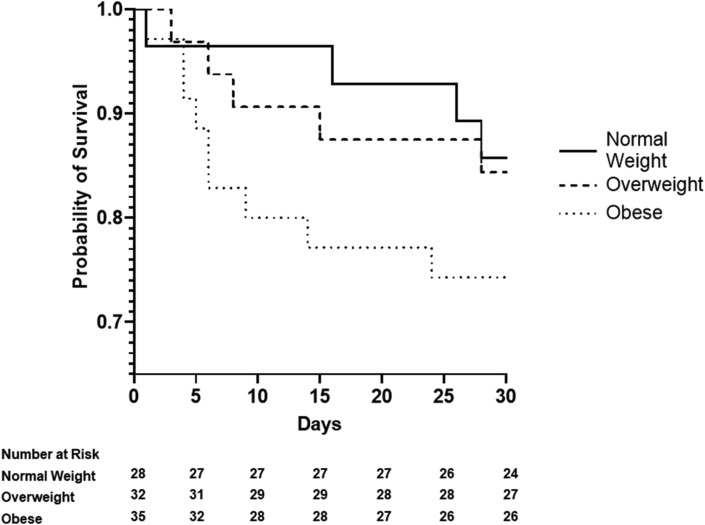
Fig. 1 shows that participants with obesity had the shorter survival at 28 days from ICU admission as compared to normal weight subjects. Estimates derived from the Cox proportional hazard models (Table 2 ), adjusted for age, gender and comorbidity, confirmed the results of the survival analysis (HR 5.30, 95%C.I. 1.26–22.34). On the contrary overweight was not associated with increased risk. In the full adjusted model age was independently associated with mortality (HR 1.13, 95%C.I 1.05–1.21).
Figure 1.
Kaplan–Meier survival curves for all-cause mortality according to BMI groups, normal weight, overweight and obese. BMI: body mass index.
Table 2.
Mortality Risk According to BMI groups, using normal weight as Reference Group.
| N of participants | Events (%) | Model 1 (unadjusted) |
Model 2 (Age and sex adjusted) |
Model 3 (Fully adjusteda) |
||||
|---|---|---|---|---|---|---|---|---|
| HR | C.I. (95%) | HR | C.I. (95%) | HR | C.I. (95%) | |||
| Normal weight | 28 | 4 (14.3) | 1 | – | 1 | – | 1 | – |
| Overweight | 32 | 5 (15.6) | 1.13 | 0.30–4.22 | 1.37 | 0.36–5.21 | 1.42 | 0.32–6.28 |
| Obese | 35 | 9 (25.7) | 1.99 | 0.61–6.49 | 3.47 | 1.00–12.03 | 5.30 | 1.26–22.34 |
HR: hazard ratio.
Adjusted for age, sex, smoking habit, hypertension, diabetes, congestive heart failure, chronic renal failure, immunodepression, cancer, chronic obstructive pulmonary disease, coronary heart disease.
In an alternative model, in which the interaction term BMI x age was added, the interaction term, was not significantly associated with mortality (OR 1.01 [95%CI: 0.99–1.03]).
Similarly, when the interaction term BMI x sex was added, both BMI and the interaction term were significantly associated with mortality (OR 1.34 [95%CI: 1.14–1.57] and 1.55 [95%CI: 1.081–2.215], respectively).
After excluding individuals who died in the first 28 days since ICU admission, obese subjects showed longer hospital and intensive care stay as compared with normal weight counterpart.
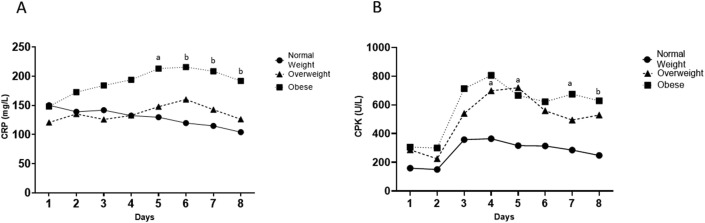
From day 5 to day 8 CRP values were significantly higher in the group of subjects with obesity as compared with the normal weight counterpart (Fig. 2 A).
Figure 2.
Changes during the first 7 days from admission in ICU in CRP level (Panel A) and CPK level (Panel B) across different BMI groups. a p < .05, b p < .01.
Subjects with obesity showed significantly higher CPK values from day 7 and day 8 and overweight higher values at admission, day 4 and day 5 as compared with normal weight subjects, even after adjustment for body weight (Fig. 2B). The threshold for BMI associated with a higher risk of mortality in ICU was calculated locating the point on the ROC curve that maximizes the sum of sensitivity and specificity (i.e. Youden's index). This cutoff associated with sensitivity of 0.611 and specificity of 0.519 was 29.23 kg/m2.
Discussion
Our study shows that in COVID-19 critically ill subject obesity (BMI≥30) is associated with a 5 times higher risk of mortality and longer hospital stay. During the first 7 days in ICU subjects with obesity showed increased CRP and CPK levels as compared with normal weight subjects.
Our result that patients with obesity are at higher risk of death as compare with normal weight is in line with Fezeu et al. and Louie et al. showing that severe obesity resulted in a two-time greater risk of death upon influenza A virus infection and hospitalization due to the infection, with moderate obesity also increasing the risks [21,22].
When the study population was further divided according to BMI categories (normal weight, overweight and obese), subjects with obesity showed higher hospital and ICU length of stay.
Previous reports on hospitalized patients observed that higher BMI is associated with increased morbidity and LOS [23,24], which is one of the major determinants of hospital costs [25].
We found that subjects with obesity have a longer ICU stay than those with normal weight.
This confirms previous studies showing that in H1N1 influenza A virus pandemic in 2009 obesity increased both the length of ICU stay and the need for mechanical ventilation [26].
Preliminary reports on SARS-CoV-2 spread showed that obese subjects are at greater risk for ICU admission and invasive mechanical ventilation [27], but the potential mechanisms that explain why obesity is associated with increased COVID-19 severity have not been determined.
We found that during the first week in ICU, CRP levels were higher in subjects with obesity when compared with normal weight subjects. COVID-19 is characterized by a reduction of T-Lymphocytes, involving in particular CD4+ and CD8+ lines with concomitant 4 times increased mortality when CD8+ level is below 75 cells/uL [28]. De Biasi et al. [28] recently showed that Covid-19 is characterized by a “cytokine storm” with increase in TNF-alfa, CCL4, CD 27, CD 40, IL-2, IL-4, IL-7, IL 8, IL-10, IL-15, Il-17A, but the greatest increase compared to controls has been observed for IL-6. Obesity is associated with a state of low grade chronic inflammation that contributes to the onset of metabolic disease, type 2 diabetes, and dyslipidemia in particular and can determine a dampened immune response to infectious agents, leading to poorer outcomes post-infection [29,30].
Obesity has been also associated with higher circulating IL-6 and CRP levels [25,26]. Adipose tissue, in particular visceral fat, has been recognized to be able to secrete great amounts of inflammatory cytokines [31].
Therefore, together, SARS-CoV-2 infection and obesity may lead to a dysregulated immune response and increased viral shedding, which could impact the outcome of COVID-19 patients [32].
In both, human and murine models, it has been shown that obese subjects present increased viral replication process, respiratory epithelium damage, pulmonary oedema, increased inflammatory response, immunopathology and poor wound recovery as compared with lean subjects, resulting in increased mortality [33,34]. Although there are still many unanswered questions regarding the pathogenesis of COVID-19 pulmonary complications, a central role of adipose tissue in the dysregulation of the immune system can be hypothesized.
Adipocytes and adipocyte like cells, such as pulmonary lipofibroblasts, may play a role in the pathogenetic response to SARS-COV2 and be responsible for the extensive pulmonary fibrosis and hypercoagulopathy observed in COVID-19 patients [31,35,36].
Normal weight and obese subjects showed similar trend in CPK level in the first 5 days from admission to ICU, but interestingly subjects with obesity showed higher levels at day 6 and 7 as compared with subjects with BMI<27.
An elevation in the serum CPK levels correlates with the extent of muscular damage and is frequently observed in a mixed ICU population [37]. Although usually asymptomatic, it could evolve into a life-threatening condition of rhabdomyolysis complicated by acute renal failure and/or disseminated intravascular coagulation [38].
In ICU unexplained CPK elevation is frequently observed in medical or infectious disease patients and elevated levels are observed in parallel with rise in LOS, independently from the APACHE II score [39].
COVID-19-associated myopathy with severe proximal and bulbar weakness, characterized by elevated levels of IL-6 and CPK, histological perivascular inflammatory infiltration with endomysial extension, muscle damage and regenerating fibers has been reported [40].
It could be hypothesized that this histopathological feature, associated with significant deficit of the upper and lower limbs can be determined by the exaggerated inflammatory response observed in patients with COVID-19 and that in subjects with obesity this phenomenon can be even more relevant, leading to worse clinical outcomes and higher mortality, as shown in our study population. In fact, obesity is associated with increased muscle vulnerability, characterized by higher levels of myostatin, IL-6 and macrophages, interfering with satellite cell activation and myoblast proliferation and differentiation, which are necessary steps for the muscle repair process after COVID-19-induced muscle injury [41,42].
Moreover obesity is characterized by an increase of lipid fat deposition between muscle fibers, the so-called intermuscular adipose tissue. This fat depot is a metabolically active tissue, capable of secreting inflammatory cytokines, increasing ceramides content and contributing to muscle damage in paracrine manner [43,44]. We can theorize that the close proximity to muscle fibers may impair the local muscle environment, leading to an impairment of muscle regeneration, also in critically ill COVID-19 patients.
CPK elevation observed in subjects with obesity could be a sign of muscle injury and partially explains the increased risk for ICU mortality and LOS observed in our population. In fact, patients with high CPK levels are more likely to receive invasive treatment in the ICU, so their muscle mass and strength may decrease with increasing ICU stay [37] and are less likely to be discharged home, because of hospital related independence loss. Further investigation of the true incidence, complication rate and independent risk factors for elevated CPK might be warranted.
Some limitations need to be mentioned. Firstly this is a single center observational prospective study. Secondly a limited number of subjects were enrolled, with few younger patients and very few older (very old) subjects.
The study sample size precludes meaningful exploration of the association of wasting with specific disease entities.
Thirdly, we used CRP as a nonspecific marker of inflammation. It is known that CRP responds relatively slowly to inflammatory stimuli and can be influenced by liver function, given that it is hepatically synthesized. Although strictly regulated by cytokines, mostly IL-6 and TNF-alfa [45,46], once daily CRP measurement has limited capacity to define the nature, cause, and scale of global and sustained inflammatory load. Further studies evaluating IL-6, TNF-alfa and adipokines should be conducted in order to better understand the pathogenetic link between obesity and unfavourable ICU health outcomes.
Last, BMI is a surrogate of total adiposity and to better understand the role of adipose tissue on COVID-19 severity, studies evaluating body fat distribution and consequent organ fibrosis should be implemented.
Our study shows that in COVID-19 ICU subject obesity is a risk factor for unfavourable health outcomes with higher mortality and longer hospital stay.
As the SARS-CoV-2 may continue to spread worldwide, clinicians should maintain a high level of attention in patients with obesity. Obese subjects should be carefully monitored and managed with a prompt and aggressive approach in order to prevent serious and life-threatening consequences and the increase of treatment associated costs.
Declaration of Competing Interest
None.
Acknowledgements
The Authors' responsibilities were as follows—LG, EP, KD: designed the research; EP, LG, KD, VS, MT: conducted research, AR, LG: analyzed the data or performed statistical analysis. AR, LG: had primary responsibility for final content, AR, LG, KD, EP, MZ, RN: wrote the paper.
Handling Editor: A. Siani
References
- 1.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospitalization and critical illness among 4,103 patients with Covid-19 disease in New York City. Br Med J. 2020;22:369. [Google Scholar]
- 5.Di Castelnuovo A., Bonaccio M., Costanzo S., Gialluisi A., Antinori A., Berselli N. COvid-19 RISk and Treatments (CORIST) collaboration. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30:1899–1913. doi: 10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden C.L., Fakhouri T.H., Carroll M.D., Hales C.M., Fryar C.D., Li X. Prevalence of obesity among adults, by household income and education - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2017;66(50):1369–1373. doi: 10.15585/mmwr.mm6650a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques Adilson, Peralta M., Naia A., Loureiro N., Gaspar de Matos M. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur J Public Health. 2018;28:295–300. doi: 10.1093/eurpub/ckx143. [DOI] [PubMed] [Google Scholar]
- 9.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatraju P.K., Ghassemieh J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the Seattle region-Case Series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. Lille Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates c., Morton C.E. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albashir A.A.D. The potential impacts of obesity on COVID-19. Clin Med. 2020;20:e109–e113. doi: 10.7861/clinmed.2020-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajifathalian K., Kumar s., Newberry C., Shah S., Fortune B., Krisko T. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York city. Obesity. 2020;28:1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Int Guid. April 7, 2020 [Google Scholar]
- 16.Simundic A.M., Bölenius K., Cadamuro J., Church S., Cornes M.P., van Dongen-Lases E.C. Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56:2015–2038. doi: 10.1515/cclm-2018-0602. [DOI] [PubMed] [Google Scholar]
- 17.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. Apache II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 18.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A.P., Zanandrea V., Zoico E., Zanardo M., Caliari C., Confente S. Inflammation and nutritional status as predictors of physical performance and strength loss during hospitalization. Eur J Clin Nutr. 2016;70:1439–1442. doi: 10.1038/ejcn.2016.159. [DOI] [PubMed] [Google Scholar]
- 20.SPSS Inc. SPSS-X user's guide. ed. 2. Mc Graw-Hill; New York: 1986. [Google Scholar]
- 21.Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 22.Louie J.K., Jean C., Acosta M., Samuel M.C., Matyas B.T., Schechter R. A review of adult mortality due to 2009 pandemic (H1N1) influenza A in California. PloS One. 2011;6 doi: 10.1371/journal.pone.0018221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akinyemiju T., Meng Q., Vin-Raviv N. Association between body mass index and in-hospital outcomes, Analysis of the nationwide inpatient database. Medicine. 2016;95:e4189. doi: 10.1097/MD.0000000000004189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philbin E., McCullough P., Dec G., Disalvo T.G. Length of stay and procedure utilization are the major determinants of hospital charges for heart failure. Clin Cardiol. 2001;24:56–62. doi: 10.1002/clc.4960240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichard C., Kyle U.G., Morabia A., Perrier A., Vermeulen B., Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with increased length of stay. Am J Clin Nutr. 2004;79:613–618. doi: 10.1093/ajcn/79.4.613. [DOI] [PubMed] [Google Scholar]
- 26.Martin E.T., Archer C., McRoberts J., Kulik J., Thurston T., Lephart p. Epidemiology if severe influenza outcomes among adult patients with obesity in Detroit, Michigan, 2011. Influenza other Respir Viruses. 2013;7:1004–1007. doi: 10.1111/irv.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A. Association of obesity with disease severity among patients with COVID-19. Obesity. 2020 doi: 10.1002/oby.22859. April 30 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Biasi S., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with Covid-19 pneumonia. Nat Commun. 2020;11:3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhurandhar N.V., Bailey D., Thomas D. Interaction of obesity and infection. Obes Rev. 2015;16:1017–1029. doi: 10.1111/obr.12320. [DOI] [PubMed] [Google Scholar]
- 30.Sattar N., McInnes I.B., McMurray J.J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 31.Smith A.G., Sheridan P.A., Harp J.B., Beck M.A. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 32.Kim J., Nam J. Insight into the relationship between obesity-induced low-level chronic inflammation and COVID-19 infection. Int J Obes. 2020;44:1541–1542. doi: 10.1038/s41366-020-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien K.B., Vogel P., Duan S., Govorkova E.A., Webby R.J., McCullers J.A. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis. 2012;205:252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruglikov I.L., Scherer P.E. The role of adipocytes and adipocytes-like cells in the severity of COVID-19 infections. Obesity. 2020;28:1187–1190. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazzaruso C., Paolozzi E., Valenti C., Brocchetta M., Naldani D., Grignani C. Association between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr Metab Cardiovasc Dis. 2020;30:1914–1919. doi: 10.1016/j.numecd.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasquarelli-do-Nascimento G., Braz-de-Melo H.A., Faria S.S., Santos I.O., Kobinger G.P., Magalhães K.G. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol (Lausanne) 2020;11:530. doi: 10.3389/fendo.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 38.Batt J., Dos Santos C.C., Herridge M.S. Muscle injury during critical illness. JAMA. 2013;310:1569–1570. doi: 10.1001/jama.2013.278482. [DOI] [PubMed] [Google Scholar]
- 39.Assanangkornchai N., Akaraborworn O., Kongkamol C., Kaewsaengrueang K. Characteristics of trauma patients with creatine kinase elevation. Crit Care. 2015;19:282. doi: 10.6705/j.jacme.2017.0702.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H., Charmchi Z., Seidman R.J., Anziska Y., Velayudhan V., Perk J. COVID-19- associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62:E53–E62. doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hittel D.S., Berggren J.R., Shearer J., Boyle K., Houmard J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58:30–38. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodell P.W., Kodesh E., Haddad F., Zaldivar F.P., Cooper D.M., Adams G.R. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol (1985) 2009;106:443–453. doi: 10.1152/japplphysiol.90831.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamboni M., Gattazzo S., Rossi A.P. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. 2019;10:5–6. doi: 10.1007/s41999-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 44.Beasley L.E., Koster A., Newman A.B., Javaid M.K., Ferrucci L., Kritchevsky S.B. Inflammation race and gender differences in computer tomography-measured adipose depots. Obesity. 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;15:74–80. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 46.Ridker P.M. C reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]