Abstract
Although some evidence showed the activation of complement systems in COVID-19 patients, proinflammatory status and lectin pathway remain unclear. Thus, the present study aimed to demonstrate the role of MBL and ficolin-3 in the complement system activation and compared to pandemic Influenza A virus H1N1 subtype infection (H1N1pdm09) and control patients. A total of 27 lungs formalin-fixed paraffin-embedded samples (10 from H1N1 group, 6 from the COVID-19 group, and 11 from the control group) were analyzed by immunohistochemistry using anti-IL-6, TNF-alfa, CD163, MBL e FCN3 antibodies. Genotyping of target polymorphisms in the MBL2 gene was performed by real-time PCR. Proinflammatory cytokines such as IL-6 and TNF-alpha presented higher tissue expression in the COVID-19 group compared to H1N1 and control groups. The same results were observed for ICAM-1 tissue expression. Increased expression of the FCN3 was observed in the COVID-19 group and H1N1 group compared to the control group. The MBL tissue expression was higher in the COVID-19 group compared to H1N1 and control groups. The genotypes AA for rs180040 (G/A), GG for rs1800451 (G/A) and CC for rs5030737 (T/C) showed a higher prevalence in the COVID-19 group. The intense activation of the lectin pathway, with particular emphasis on the MBL pathway, together with endothelial dysfunction and a massive proinflammatory cytokines production, possibly lead to a worse outcome in patients infected with SARS-Cov-2. Moreover, 3 SNPs of our study presented genotypes that might be correlated with high MBL tissue expression in the COVID-19 pulmonary samples.
Abbreviations: ACE-2, Angiotensin-Convertase-Enzyme-2 Receptors; DAD, Diffuse Alveolar Damage; FFPE, Formalin-Fixed Paraffin-Embedded; H1N1pdm09, Pandemic Influenza A Virus H1N1 Subtype Infection; H&E, Hematoxylin and Eosin; MBL, Mannose-Binding Lectin; HPF, High-Power Field; rRT-PCR, Reverse Transcriptase-Polymerase Chain Reaction; IHC, Immunohistochemistry
AT A GLANCE COMMENTARY.
Malaquias M. A. S., et al.
Background
Efforts are underway with tests, including antiviral drugs, anti-inflammatory agents, clinical trials for blocking cytokines/chemokines, and complement/coagulation/bradykinin pathways. Interestingly, the complement system activation mechanisms received particular attention to identifying new biomarkers for combinatorial immunotherapy approaches.
Translational Significance
The COVID-19 higher tissue expression of proinflammatory cytokines, inflammatory adhesion molecules, and complement related-proteins with a higher prevalence of MBL gene polymorphisms may suggest the role of the lectin pathway activation in SARS-CoV-2 pathogenesis. Together with the endothelial dysfunction and cytokine storm, the complement activation could be a pathway linking severe lung injury and systemic immunothrombosis to fatal outcomes.
Alt-text: Unlabelled box
INTRODUCTION
The SARS-CoV-2 is unique considering some virus notable peculiarities, such as its high affinity for widely expressed angiotensin-convertase-enzyme-2 receptors (ACE-2), its wide capacity to spread quickly to all nations, its low immunogenic surface proteins with delayed adaptive immune response, its high concentration in aerosols and wide viability in the external environment and finally, the long incubation time, the large number of asymptomatic patients and the similarity of early symptoms to the common flu.1 , 2 The most disturbing points are the clinical progression of the COVID-19 and its physiopathology mechanism that may not be aggravated exclusively by the virus infection but also by the immune-inflammatory process characterized by a massive cytokine production.3, 4
Notably, the SARS-CoV-2 presents several unique glycosylation sites suggesting a different shielding or glycan camouflage pattern of the spike proteins, which may trigger variances in host immunity.5 These glycosylated proteins might induce the complement activation, triggering a proinflammatory response.6 , 7 The lectin pathway of the complement comprises the mannose-binding lectin (MBL), collectin 11 (CL-K1) and ficolins (Ficolin-1, Ficolin-2, and Ficolin-3) that are responsible for carbohydrate recognition on the microorganism surface. After the glycan-binding, the activated lectin forms complexes with the serine proteases associated with MBL 1 and 2 (MASP-1 and MASP-2), which they cleave C4 and C2 and form C3 convertase (C4b2a).8
The complement component C3 was required for protection from Influenza A virus H1N1 subtype infection collaborating with viral clearance and regulating lung inflammatory process.9, 10, 11 Immunohistochemistry (IHC) of COVID-19-patient lung tissue showed stronger staining signal for MBL, MASP-2, C4alpha, C3 or C5b-912 and in renal tubular cells was observed the complement C5b-9 deposition mediating tubular pathogenesis associated with COVID-19.13 Taken together; these results were suggesting that complement pathways might be hyperactivated in COVID-19 patients.
Several common polymorphisms in the MBL2 gene that cause a reduction of serological MBL levels, as well as dysfunctional proteins have been described.14 Individuals with MBL deficiency exhibit increased vulnerability to both infectious diseases,15 and autoimmune diseases, such as rheumatoid arthritis,16 systemic lupus erythematosus,17 and Kawasaki disease.18
Although higher levels of activated complement such as MBL, MASP-2, and C3 fragments were found in COVID-19 patients (Gao et al., 2020, Diao et al., 2020), indicating the activation of complement pathways, the relationship between inflammation and complement activation in the SARS-CoV-2 pathogenesis is not completely understood. Thus, the present study aimed to demonstrate, in COVID-19 lung samples, possible routes involved in complement activation and compared to H1N1pdm09 and Control patients. Moreover, genetic variants of MBL2 that may be associated with MBL-tissue expression, was also investigated.
METHODS
Ethical approvals
This present study was approved by the National Research Ethics Committee (Conselho Nacional de Ética em Pesquisa – CONEP) by the numbers 3.944.734/2020 (COVID-19 patients) and 2.550.445/2018 H1N1pdm09 and Control patients).
The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations. The sample collection followed all relevant ethics and safety protocols.
Families permitted the postmortem biopsy of the COVID-19, H1N1pdm09, and Control patients; and signed the informed consent forms.
Patients
H1N1 group (n = 10) comprises lung samples from postmortem biopsies of patients whose cause of death was diffuse alveolar damage (DAD) caused by pandemic Influenza A virus H1N1 subtype during the 2009 outbreak. Clinical data of 10 cases were obtained from medical records during hospitalization in the ICU at Hospital de Clínicas in Curitiba-Brazil.
COVID-19 group (n = 6) comprises lung samples from postmortem biopsies of patients whose cause of death was DAD caused by SARS-CoV-2 during the 2020 outbreak. Clinical data of 6 cases were obtained from medical records during hospitalization in the ICU at Hospital Marcelino Champagnat in Curitiba-Brazil.
Testing for Influenza A virus H1N1 subtype and SARS-CoV-2 was performed on nasopharyngeal swabs taken during ICU hospitalization, and real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) was positive in all cases.
Control group (n = 11), composed of lung samples from necropsies of patients who died due to various causes, not involving lung lesions, was added in order to verify the basal tissue concentration of IL-6, TNFα, CD163, MBL e FCN3 in lung samples without pathological alterations.
Immunohistochemical assays
Minimally invasive lung biopsy was performed through a left anterior mini thoracotomy with upper left lobe lingular segment resection. The resected pieces were 3 × 3 cm. The samples were formalin-fixed, paraffin embedded (FFPE) and stained with hematoxylin and eosin (H&E) to find the properly areas for the IHC techniques.
The FFPE lung samples were also performed by IHC reactions using primary monoclonal antibodies anti IL-6 (Monoclonal/Mouse, Ab9324, 1:400, Abcam), TNFα (Policlonal/Rabbit, Ab6671, 1:100, Abcam), CD163 (Policlonal/Rabbit, 14215, 1:1000, Thermo Fisher), FCN3 (Policlonal/Rabbit, Orb30580, 1:600, Biorbyt), and MBL (Policlonal/Rabbit, PAB480Hu02, Cloud-Clone, 1:200).
The slides of IL-6, TNFα, CD117, FCN3, and MBL were scanned on the Axio Scan Scanner Z1 (Carl Zeiss, Germany). Afterward, 10 high-power field (HPF) were selected of each sample and the positive areas were quantified using Image-Pro Plus software version 4.5 (Media Cybernetics, USA). Subsequently, these areas were converted into percentage to enable statistical analysis.
CD163 slides were also used to score macrophages, in 30 HPF. The HPF was chosen randomly from the septum and lumen alveolar, where the macrophages were scored.
Genetic extraction and amplification
The DNA was obtained from FFPE samples using a commercially available paraffin DNA extraction kit (Qiagen). After determining the concentration, the samples will be diluted to a final concentration of 20 ng/µL, for working solution, and stored in a freezer at –20°C with restricted access.
The polymorphisms in the proposed candidate genes were chosen by criteria of functional relevance in articles published in qualified journals and confirmed in the SNPinfo NIH site (SNPinfo, 2020). The samples purified DNA was amplified by real-time PCR (Applied Biosystems 7500 Real-Time PCR System).
Statistical analysis
The quantitative variables were described by means and standard deviation, and by medians with minimum and maximum values. Normality condition of the variables in each group was evaluated using the Shapiro-Wilks test. Kruskal-Wallis test for comparison with quantitative variables. Data were analyzed using the computer program The R Project for Statistical Computing. Data were analyzed by IBM SPSS Statistics v.20.0 software.
RESULTS
Patients' baseline
The clinic and demographic data were described in Tables I and II . The mean age was significant (P = 0.0048) higher in the COVID-19 group compared to the H1N1 group. The same aspect was observed between COVID-19 and Control groups (P = 0.0026) for mean age. Survival days in the COVID-19 group were significant (P = 0.0058) higher than in the H1N1 group. The same data present significant differences (P = 0.0109) when comparing the COVID-19 and Control groups. Mechanical ventilation time was no significantly different (P = 0.1851) between COVID-19 and H1N1 groups.
Table I.
Baseline samples COVID-19
| Cases | Gender/age | Comorbidities | Mechanical ventilation | Time from hospitalization to death |
|---|---|---|---|---|
| 1 | Female, 87 years old | Systemic arterial hypertension dyslipidemia hypothyroidism | ||
| Senile dementia | 6 days | 6 days | ||
| 2 | Male, 53 years old | Class II obesity | 10 days | 12 days |
| 3 | Female, 85 years old | Systemic arterial hypertension brain stroke vascular dementia | 0 days | 23 days |
| 4 | Male, 73 years old | Type 2 diabetes mellitus chronic kidney disease dialysis atrial fibrillation coronary disease heart failure peripheral obstructive artery disease | 10 days | 38 days |
| 5 | Male, 80 years old | Systemic arterial hypertension coronary disease heart failure Class III obesity | 21 days | 23 days |
| 6 | Male, 81 years old | Systemic arterial hypertension chronic kidney disease dialysis brain stroke | 8 days | 8 days |
Table II.
Comparative table between COVID-19, H1N1, and CONTROL groups for gender, age, survival in days, mechanical ventilation in days and immunohistochemical expression of inflammatory biomarkers
| Variables | Covid-19 (n = 6) | H1N1 (n = 10) | P-value‡ | Control (n = 11) | P-value§ |
|---|---|---|---|---|---|
| Gender | Male 4 (66.6%) | Male 8 (80.0%) | 0.5510 | Male 8 (72.7%) | 0.7933 |
| Female 2 (33.4%) | Female 2 (20%) | Female 3 (27.3%) | |||
| Age (years)* | 80.5 (53–87) | 44 (23–61) | 0.0048 | 45 (18–60) | 0.0026 |
| 76.5±12.5 | 43.5±13.9 | 42.3±14.3 | |||
| Time from hospitalization to death (days)* | 18 (6–38) | 1.5 (1–19) | 0.0058 | 4(1–46) | 0.0109 |
| 18.8±11.6 | 4.7±6.1 | 7.6±13.1 | |||
| Mechanical ventilation* | 8 (0-21) | 1.5 (1–19) | 0.1851 | — | — |
| 9.2±6.8 | 4.7±6.1 | — | |||
| CD163 score*,† | 52.9 (14.4–87.8) | 71.4 (37.1–71.4) | 0.1931 | 31.5 (19.5–47.6) | 0.3149 |
| 49.0±28.7 | 69.2±20.3 | 31.7±8.2 | |||
| IL-6 tissue expression in percentage per HPF* | 3.9 (0.3–7.6) | 1.3 (0.7–3.1) | 0.0652 | 0.0 (0.0–5.6) | 0.0049 |
| 4.0±2.5 | 1.6±0.9 | 0.6±1.6 | |||
| TNF-alpha tissue expression in percentage per HPF* | 5.8 (3.2–35.3) | 2.1 (0.1–4.9) | 0.0092 | 2.2 (0.2–4.1) | 0.0048 |
| 12.3±12.9 | 2.5±1.5 | 1.9±1.5 | |||
| ICAM-1 tissue expression in percentage per HPF* | 4.3 (1.4–20.6) | 0.9 (0.0–3.3) | 0.0034 | 0.4 (0.1–2.6) | 0.0034 |
| 7.4±7.3 | 1.0±1.0 | 0.8±0.8 | |||
| FCN-3 tissue expression in percentage per HPF* | 2.3 (0.0–11.9) | 1.5 (0.3–7.6) | 0.8283 | 0.1 (0.0–0.7) | 0.0652 |
| 3.8±4.6 | 2.5±2.3 | 0.2±0.2 | |||
| MBL tissue expression in percentage per HPF* | 5.4 (0.2–13.3) | 0.1 (0.0–1.2) | 0.0126 | 0.1 (0.0–1.9) | 0.0170 |
| 6.3±6.6 | 0.2±0.4 | 0.5±0.7 |
Underlined values minimum and maximum number for the biomarker.
Median (Min-Max) - Average ± SD.
Mean number of CD163+ macrophages in 30 high-power field (HPF).
P-values obtained were compared between COVID-19 versus H1N1.
P-values obtained were compared between COVID-19 and CONTROL group. p-values were performed using the non-parametric Kruskal-Wallis test.
Pathological features
The histological aspects observed in the COVID-19 cases were diffuse proliferative alveolar damage, type 2 pneumocyte hyperplasia, hyaline membranes with moderate alveolar edema, mild septal lymphocytic infiltration and scarce septal and intra-alveolar neutrophils recruitment. Fibrinous immune thrombosis was observed following by neutrophilic endotheliitis in areas of both damaged and more preserved lung parenchyma in all cases. Endothelial tumefaction in the alveolar capillaries is another indicator of endothelial activation. Signs of secondary bacterial pneumonia were not observed.
The histopathological features of H1N1 cases are different than observed in COVID-19. There is DAD with fewer hyaline membranes but higher septal thickness with massive septal lymphocytic infiltration and severe septal and intra-alveolar neutrophils recruitment. There is no significant endothelial activation, fibrinous thrombosis, and neutrophilic endotheliitis. Signs of bacterial coinfections were found in 80% of cases, with the presence of Haemophilus influezae (60%), Streptococcus pneumoniae (10%), and Mycoplasma pneumoniae (10%) being reported in the samples.
Immunohistochemical results
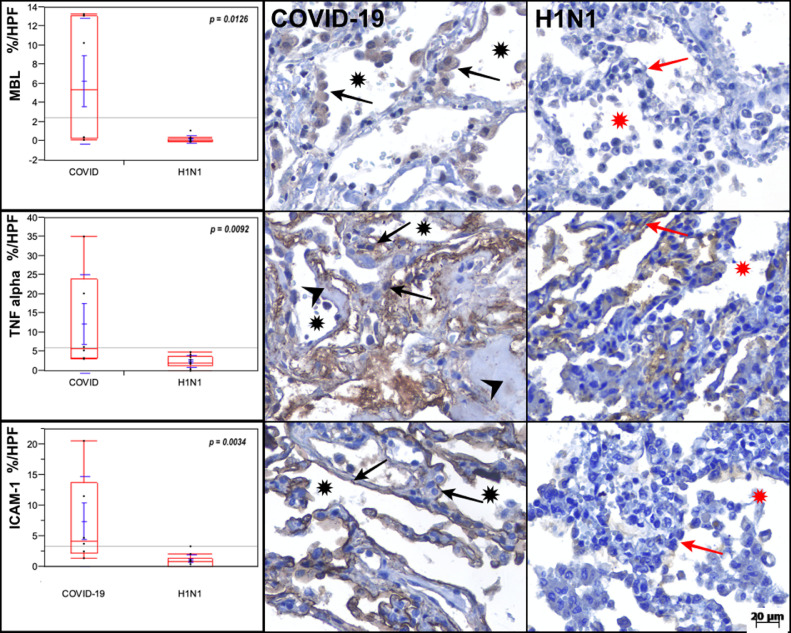
Intense tissue expression of cytokines was observed in the COVID-19 compared with H1N1 group for both IL-6 (P = 0.0652), and TNF-alpha (P = 0.0092). The Control group presented lower immunolabeling for these cytokines (IL-6 and TNF-alpha) compared to COVID-19 group (P = 0.0049 and P = 0.0048 respectively) (Fig 1 ; Table II).
Fig 1.
Graphs showing the comparison between the COVID-19, H1N1, and Control groups regarding MBL, TNF-alpha and ICAM-1 (in percentage per HPF). MBL tissue expression is higher in the pneumocytes (black arrows) of the COVID-19 case than in the H1N1 case. TNF-alpha is remarkably high in the alveolar septal cells (black arrows) of the COVID-19 case than in the H1N1 case. ICAM-1 tissue expression is higher in the pneumocytes (black arrows) of the COVID-19 case than in the H1N1 case. Alveolar lumens are identified with asterisks and hyaline membranes with arrowheads. Kruskal-Wallis test was used for P-values.
The ICAM-1 showed higher tissue expression in COVID-19 group compared to H1N1 (P = 0.0034) and control group (P = 0.0034) (Fig 1; Table II).
Regarding the proteins involved in the complement system, the FCN3 tissue expression in the COVID-19 group showed significant differences compared to H1N1 (P = 0.8283) and was higher than the Control group (P = 0.652; Table II). The MBL tissue expression in COVID-19 group was higher compared to H1N1 (P = 0.0126) and Control group (P = 0.0170) (Fig 1; Table II).
When the macrophages (CD163) in lung-tissue were scored, the COVID-19, H1N1, and Control group showed no significant differences in the number of macrophages (Table II).
Genotyping results
Genotyping distribution in all three groups can be observed in Table III , and the genotypes AA for rs180040 (G/A), GG for rs1800451 (G/A) and CC for rs5030737 (T/C) showed a higher prevalence in the COVID-19 group. Besides, these same genotypes were associated with elevated values of MBL expression (Table III).
Table III.
Genotypic analysis of the SNPs in MBL2 gene and tissue expression of MBL
| Reference SNP* and variation 1/2 | Genotypes | COVID-19 (n = 6)†‡§ |
H1N1 (n = 10)†‡§ |
CONTROL (n = 11)†‡§ |
|||
|---|---|---|---|---|---|---|---|
| rs1800450 G/A | GG | 0 (0.0) | - | 0 (0.0) | - | 0 (0.0) | - |
| GA | 2 (40.0) | 0.4 (0.4–0.5) | 6 (60.0) | 0.1 (0.0–1.2) | 5 (71.4) | 0.1 (0.0–1.2) | |
| AA | 3 (60.0) | 13.1 (10.3–13.3) | 4 (40.0) | 0.0 (0.0–0.4) | 2 (28.6) | 0.1 (0.1–0.1) | |
| rs1800451 G/A | GG | 5 (83.3) | 11.7 (0.4–13.3) | 9 (90.0) | 0.0 (0.0–0.2) | 1 (10.0) | - |
| GA | 0 (0.0) | - | 1 (10.0) | - | 0 (0.0) | - | |
| AA | 1 (16.7) | - | 0 (0.0) | - | 9 (90.0) | 0.1 (0.0–0.3) | |
| rs5030737 T/C | TT | 0 (0.0) | - | 0 (0.0) | - | 1 (10.0) | - |
| TC | 0 (0.0) | - | 0 (0.0) | - | 3 (30.0) | 0.1 (0.1–0.3) | |
| CC | 6 (100.0) | 10.3 (0.4-13.3) | 10 (100.0) | 0.0 (0.0–1.2) | 6 (60.0) | 0.0 (0.0–0.1) | |
SNP identifier based on NCBI dbSNP.
Some individuals may have failure in genotyping the marker.
Tissue expression of MBL was presenting by Median (Minimum-Maximum).
Genotype was expressed by number and percentage.
DISCUSSION
It is known that SARS-CoV-2 contains nonconserved glycosylation sites suggesting a different pattern of spike protein that can induce an immune system exacerbated response.5 The complement system activation through lectin pathway by the interaction of MBL and FCN3 with carbohydrates or acetylated residues, respectively present on the surface of pathogens or necrotic cells, promotes the expression of adhesion molecules, the secretion of proinflammatory and chemotaxis molecules and the leukocyte activation.19 The N-linked glycosylation site N330 in the SARS-CoV spike (S) protein has been described as being critical for specific interactions with MBL.20 Although the activation of the complement pathways in patients with SARS has been indicated by the higher levels of C3 a and C4a deposit,21 , 22 the mechanism of complement system activation induced by SARS-CoV-2 is not well understood. The present study investigated the involvement of the complement system activation by the lectin pathway in COVID-19 and H1N1pdm09 diseases.
Higher tissue expression of MBL was observed in the COVID-19 group compared to the others (Table II; Fig 1) with increased FCN-3 protein in both pandemic viral infections compared to the control samples. These observations show the possible recognition of glycoproteins from the viral envelope through MBL and ficolin, which culminates in the activation of the complement through MASP-1 and MASP-2.8 While glycosylation of the viral envelope proteins may promote a barrier against the development of antiviral antibodies, also provides immune recognition through MBL and ficolins binding.23 It is known that low concentrations of MBL are associated with recurrent infections in childhood, especially in combination with other states of immunodeficiency due to defects in microorganism opsonization.24 On the other hand, high concentrations of MBL can be harmful by facilitating infections by intracellular microorganisms and aggravating tissue damage by complement system activation.25, 26, 27
Although several clinical studies are associating COVID-19 with an increased risk of endotheliopathy and immunothrombosis,28 , 29 the pathophysiological mechanisms remain unclear. It is known that the massive complement activation can increase the production of potent anaphylatoxins C3a and C5a that may intensify immunothrombotic events by inducing mast cell degranulation, initiating cytokine storms and promoting vascular permeability and these mechanisms have been demonstrated in the endothelial dysfunction associated with COVID-19.30, 31, 32, 33, 34 Besides, the activation of the lectin pathway plays an important role in the coagulation cascade activation through the binding of MBL or ficolins to fibrinogen or fibrin.35 Furthermore, the endothelial activation caused by hyperinflammation status, supported by higher tissue expression of IL-6, TNF-alpha and ICAM-1 in COVID-19 group than H1N1 (P = 0.0652, borderline; P = 0.0092; P = 0.0034, respectively) and Control groups (P = 0.0049, P = 0.0048; P = 0.0034, respectively), provides procoagulant status.36 In addition, the C5b-9 terminal complement complex may induce endothelial activation triggering the expression of adhesion molecules and platelet-activating chemokines. Together, these factors may promote proinflammatory response, endotheliopathy, and vascular permeability impairment triggering the immunothrombotic process.37 The COVID-19 patients’ clinical data of our study (Supplementary Table) suggest, by the presence of thrombocytopenia and high D-dimer, a coagulation cascade hyperactivation. Our results also suggest a crucial role for endothelial dysfunction in this process. While the H1N1 group, no significant endothelial activation was observed, fibrinous thrombosis and neutrophilic endotheliitis were associated with SARS-Cov-2 infection.38, 39, 40
The consequences of complement activation during SARS-CoV-2 infection can be considered dubious. On the one hand, it can be protective when recognizing glycosylated proteins on the microorganism surface and inducing proinflammatory response; but on the other hand, the complement system hyperactivation could result in an intense proinflammatory and hypercoagulation status and consequently severe tissue damage. Corroborating these observations, a recent pre-print report of lung biopsy from patients with severe and fatal SARS-Cov-2 infection showed extensive complement activation, characterized by the deposition of C3 fragments.41 Another preprinted study suggested that the MBL opsonization may be necessary for the neutralization of SARS-Cov-2.12 Furthermore, lung biopsy of severe COVID-19 patients revealed that alveolar-capillary endothelium lesions were accompanied by extensive deposits of the C5b-9 terminal complex.42 , 43
The present study did not observe secondary bacterial infection in the cases of COVID-19, while it was found in 80% of H1N1 cases. The incidence of secondary bacterial infections in COVID-19 appears to be low, related to the severity of the lung injuries and the last of ICU admission.44 As the complement system acts mainly at the beginning of bacterial infections, and the susceptibility to infections is associated with MBL deficiency, the early complement system hyperactivation in the COVID-19 patients might be protective considering this complication.45
The high MBL tissue expression might be associated with 3 specifics MBL2 genotypes (rs1800450 AA; rs1800451 GG; rs5030737 CC) in the COVID-19 group (Table III) and might be responsible for the complement system hyperactivation and low bacterial coinfection prevalence in these patients.46 Literature shows the MBL2 polymorphisms can be associated with low MBL expression and predisposition for infectious diseases.47 However, a study with Egyptian patients and rheumatic fever found high MBL expression associated with a higher prevalence for the AA genotype of the MBL2, rs1800450 gene.48 Corroborating with this data, 3 SNPs of our study presented genotypes that might be associated with high MBL tissue expression in the pulmonary alveolar microenvironment.
The main limitations of this study are the small number of cases and data-based postmortem samples that can only provide a piece of static information at the time of the death. A limitation that might be influencing the expression of biomarkers would be the nonpairing baseline characteristics in the 3 groups (age and survival times).
Despite the excessive number of deaths resulting from the current pandemic COVID-19, very few descriptions of necropsies with genotyping and IHC analysis have been available to the scientific community. Here, we described the massive activation of the lectin pathway, with consequences of endothelial dysfunction and the proinflammatory response that possibly driving the worst outcome in these patients. Finally, we propose that some MBL2 polymorphisms might be associated with higher MBL tissue expression, suggesting that these patients could be more susceptible to hyperactivation of the complement system during SARS-CoV-2 infection.
Acknowledgments
Lúcia de Noronha is the recipient of the CNPq scholarship. Ana Carolina Gadotti is the recipient of research fellowships from Fundação Araucária/CAPES (PR, Brazil). All other authors declare no significant competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript. No grant supported the present study. All authors declare knowledge of the Translational Research's policy of the conflicts of interest and the authorship agreement.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.trsl.2020.11.008.
Appendix. Supplementary materials
References
- 1.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cossarizza A, De Biasi S, Guaraldi G, Girardis M, Mussini C. SARS‐CoV‐2, the Virus that Causes COVID‐19: cytometry and the new challenge for global health. Cytom Part A. 2020;97:340–343. doi: 10.1002/cyto.a.24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:55. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vankadari N, WuHan Wilce JA.Emerging. (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastellos DC, Reis ES, Lambris JD. Complement C5a-Mediated TAM-ing of antitumor immunity drives squamous carcinogenesis. Cancer Cell. 2018;34:531–533. doi: 10.1016/j.ccell.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Héja D, Kocsis A, Dobó J, et al. Revised mechanism of complement lectin-pathway activation revealing the role of serine protease MASP-1 as the exclusive activator of MASP-2. Proc Natl Acad Sci U S A. 2012;109:10498–10503. doi: 10.1073/pnas.1202588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez SF, Jayasekera JP, Carroll MC. Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine. 2008;26(SUPPL. 8):I86–I93. doi: 10.1016/j.vaccine.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 10.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81(7):3487–3494. doi: 10.1128/jvi.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 12.Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 doi: 10.1101/2020.03.29.20041962. . Published online. [DOI] [Google Scholar]
- 13.Diao B, Feng Z, Wang C, et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. medRxiv. 2020;2:2020. doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garred P, Larsen F, Seyfarth J, Fujita R, Madsen HO. Mannose-binding lectin and its genetic variants. Genes Immun. 2006;7:85–94. doi: 10.1038/sj.gene.6364283. [DOI] [PubMed] [Google Scholar]
- 15.Koch A, Melbye M, Sørensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. J Am Med Assoc. 2001;285:1316–1321. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 16.Dolman KM, Brouwer N, Frakking FNJ, et al. Mannose-binding lectin deficiency is associated with early onset of polyarticular juvenile rheumatoid arthritis: A cohort study. Arthritis Res Ther. 2008;10:R32. doi: 10.1186/ar2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panda AK, Parida JR, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Low producer MBL genotypes are associated with susceptibility to systemic lupus erythematosus in Odisha, India. Hum Immunol. 2013;74:114–119. doi: 10.1016/j.humimm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, rong Li C, bai Li Y, jun Huang H, xin Li R, bin Wang G. Correlation between mannose-binding lectin gene codon 54 polymorphism and susceptibility of Kawasaki disease. Zhonghua Er Ke Za Zhi. 2004;42:176–179. https://pubmed.ncbi.nlm.nih.gov/15144709/ Accessed August 5, 2020. [PubMed] [Google Scholar]
- 19.Beltrame MH, Catarino SJ, Goeldner I, Boldt ABW, Reason IJ, de M. The lectin pathway of complement and rheumatic heart disease. Front Pediatr. 2015;2(JAN):148. doi: 10.3389/fped.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Lu K, Pfefferle S, et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84:8753–8764. doi: 10.1128/jvi.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JH, Chang YW, Yao CW, et al. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc Natl Acad Sci U S A. 2004;101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang RTK, Poon TCW, Chan KCA, et al. Serum proteomic fingerprints of adult patients with severe acute respiratory syndrome. Clin Chem. 2006;52:421–429. doi: 10.1373/clinchem.2005.061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta PK, Rappaport J. HIV and complement: hijacking an immune defense. Biomed Pharmacother. 2006;60:561–568. doi: 10.1016/j.biopha.2006.07.087. [DOI] [PubMed] [Google Scholar]
- 24.Garred P, Madsen HO, Balslev U, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–240. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 25.Garred P, Thiel S, Madesn HO, Ryder LP, Jensenius JC, Svejgaard A. Gene frequency and partial protein characterization of an allelic variant of mannan binding protein associated with low serum concentrations. Clin Exp Immunol. 1992;90:517–521. doi: 10.1111/j.1365-2249.1992.tb05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garred P, Larsen F, Madsen HO, Koch C. Vol. 40. Elsevier Ltd; 2003. Mannose-binding lectin deficiency - Revisited; pp. 73–84. (Molecular Immunology). [DOI] [PubMed] [Google Scholar]
- 27.Boldt ABW, Goeldner I, De Messias-Reason IJT. Vol. 56. Academic Press Inc; 2012. Relevance of the lectin pathway of complement in rheumatic diseases; pp. 105–153. (Advances in Clinical Chemistry). [DOI] [PubMed] [Google Scholar]
- 28.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98 doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Franks TJ, Chong PY, Chui P, et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amara U, Rittirsch D, Flierl M, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632(Davis 2004):71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganter MT, Brohi K, Cohen MJ, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 35.Jensenius JC. The mannan-binding lectin (MBL) pathway of complement activation: Biochemistry, biology and clinical implications. Adv Exp Med Biol. 2005;564:21–22. doi: 10.1007/0-387-25515-X_6. [DOI] [PubMed] [Google Scholar]
- 36.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;0 doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Zhou P, Wei Y, et al. Histopathologic changes and SARS-COV-2 immunostaining in the lung of a patient with coviD-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID‐19. J Thromb Haemost. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID-19: friend and foe. JCI Insight. 2020 doi: 10.1172/jci.insight.140711. Published online June 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4:e28. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu Y, Yang Q, Xu M, et al. Secondary Bacterial Infections in Critical Ill Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winthrop KL, Mariette X, Silva JT, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobuli. Clin Microbiol Infect. 2018;24:S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Eisen DP, Minchinton RM. Impact of Mannose-Binding Lectin on Susceptibility to Infectious Diseases. Clin Infect Dis. 2003;37:1496–1505. doi: 10.1086/379324. [DOI] [PubMed] [Google Scholar]
- 47.Eisen DP, Dean MM, Boermeester MA, et al. Low serum mannose-binding lectin level increases the risk of death due to pneumococcal infection. Clin Infect Dis. 2008;47:510–516. doi: 10.1086/590006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomaa MH, Ali SS, Fattouh AM, Hamza HS, Badr MM. MBL2 gene polymorphism rs1800450 and rheumatic fever with and without rheumatic heart disease: An Egyptian pilot study. Pediatr Rheumatol. 2018;16 doi: 10.1186/s12969-018-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.