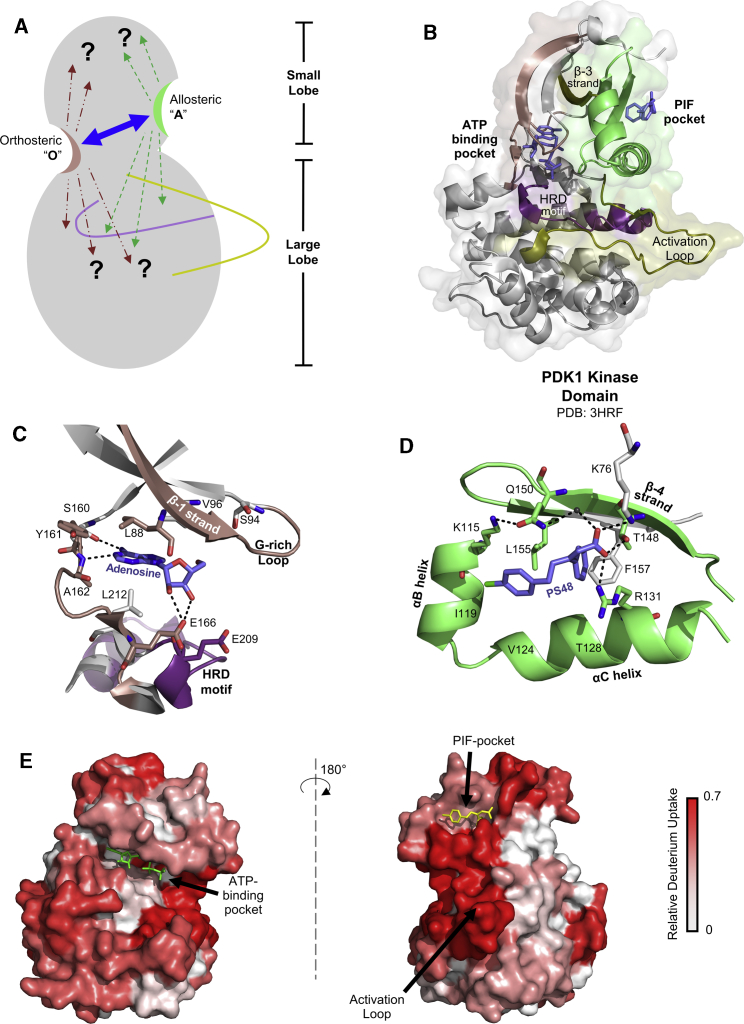
Figure 1.
Basis for allosteric crosstalk between the two noncontiguous ligand sites of PDK1 kinase. (A) Kinase domain of PDK1 in cartoon representation showing the N- and C-terminal lobes and loops critical for catalysis of phosphotransfer activity: HRD motif loop (purple) and activation loop (olive). Two ligand binding sites at the small lobe are marked “O” for the orthosteric ATP-binding pocket and “A” for its corresponding allosteric PIF-pocket. A double-headed arrow between them indicates bidirectional allostery. The interplay between PDK1 dynamics and the allosteric effects of ligand binding at either site is unknown. (B) Structure of PDK1 bound to ATP and PS48 (PDB: 3HRF (38)) showing ATP- (salmon) and PIF-pocket sites (green). Binding ligands are blue. Important secondary structure elements of the kinase are highlighted: β-1 strand, G-rich loop and the hinge region at the ATP-binding pocket (salmon), the HRD motif loop (purple), β-3 strand and activation loop (olive), and PIF-pocket (αB, αC, and β-4) (green). Shown is a close-up of ATP- (C) and PIF-pocket (D), with important orthosteric contacts at the ATP-pocket (using adenosine-bound PDK1 (PDB: 5LVN)) and PIF-pocket (using PDK1-ATP-PS48 complex structure (PDB: 3HRF)). (E) Deuterium exchange heatmaps showing relative fractional deuterium uptake (data uncorrected for back exchange) in apo-PDK1 ranging from 0 to a maximum of 0.7 (PDB: 3HRF). Shown is a 180° view of apo-PDK1 heatmap at deuterium exchange time (t = 1 min). Ligands are shown only to demarcate the two pockets. To see this figure in color, go online.