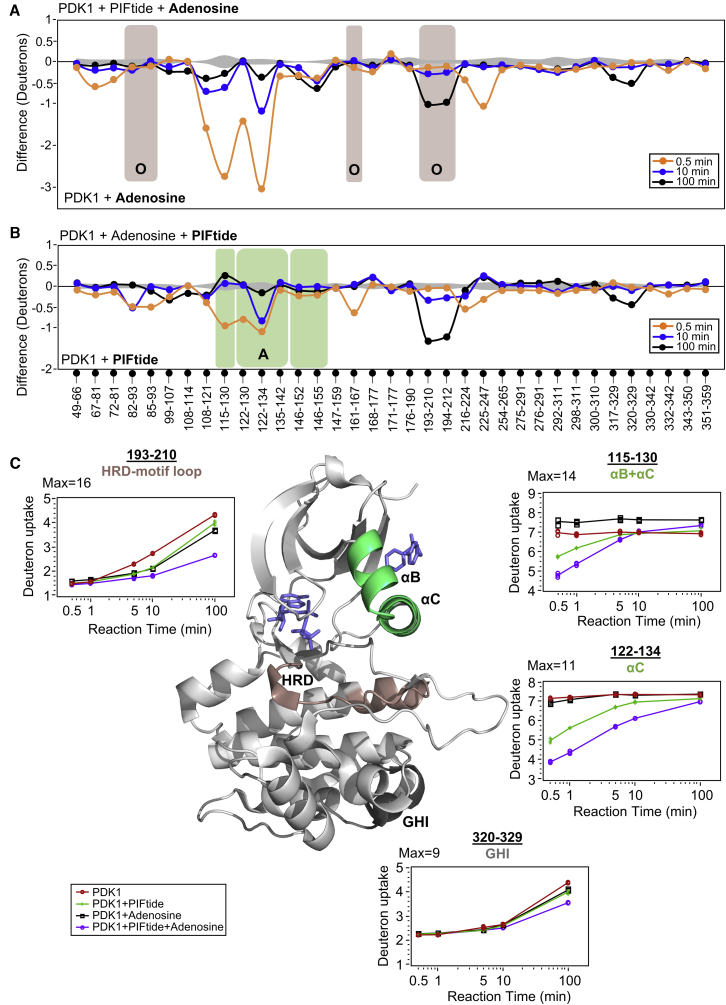
Figure 4.
Combinatorial allostery in PDK1 upon ligand binding at both ATP- and PIF-pockets. (A–B) Deuterium exchange difference plots of PDK1 bound to both PIFtide and adenosine relative to singly liganded PDK1. Axes, time points, error curves, and highlighted regions of interest are as per Fig. 2A. Decreased deuterium exchange at 193–212 (ATP-pocket, salmon) in (A) and 115–134 (PIF-pocket, green) in (B) reflect cooperatively enhanced binding of adenosine and PIFtide, respectively, in the presence of the corresponding cooperative ligand. (C) Semilog deuterium uptake plots for representative peptides that show enhanced protection from deuterium exchange in presence of both ligands. Red, green, black, and purple plots represent deuterium exchange in apo-, PIFtide-bound, adenosine-bound, and both (adenosine + PIFtide)-bound PDK1, respectively, as indicated in the key. Structure of PDK1-ATP-PS48 complex (PDB: 3HRF) in the center highlights the loci revealing combinatorial allosteric effects. See also Fig. S3; Tables S3 and S4. To see this figure in color, go online.