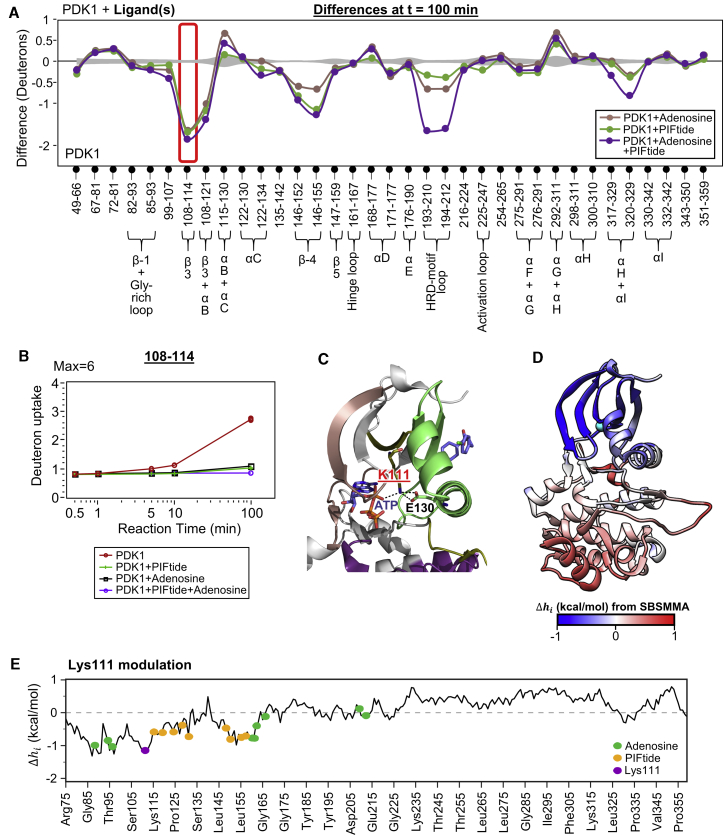
Figure 6.
Lys111 integrates allosteric signals from both ATP- and PIF-pockets. (A) Difference plot comparing protein-wide differences in deuterium exchange between ligand-bound and apo PDK1 at t = 100 min, with indicated ligand(s) bound as per key. Axes and error curves are similar to the difference plots in Fig. 2. Peptide 108–114, highlighted in red box, spans the β-3 strand and shows significant protection only at 100 min. (B) Semilog deuterium exchange plot of the peptide 108–114 in apo, adenosine-, and PIFtide-bound states, as shown in key below. Presence of either ligand decreases exchange in the peptide after 100 min. (C) A network of interactions between the conserved Lys111 on β-3 strand and conserved Glu130 of αC helix of the PIF-pocket, as well as the α-phosphate of ATP in PDK1 (PDB: 3HRF). (D) Heatmap of the allosteric configurational work due to Lys111 modulation, as calculated using SBSMMA, with blue indicating negative , whereas red is positive. (E) A plot of Δhi output from SBSMMA for Lys111 perturbation, with along y axis and PDK1 residues along x axis. See also Tables S3 and S4. To see this figure in color, go online.