Extended Data Fig. 8 |. Immunoblot characterization of virion capsids of VZV and HSV-1 after Triton X-100 lysis and KCl treatment.
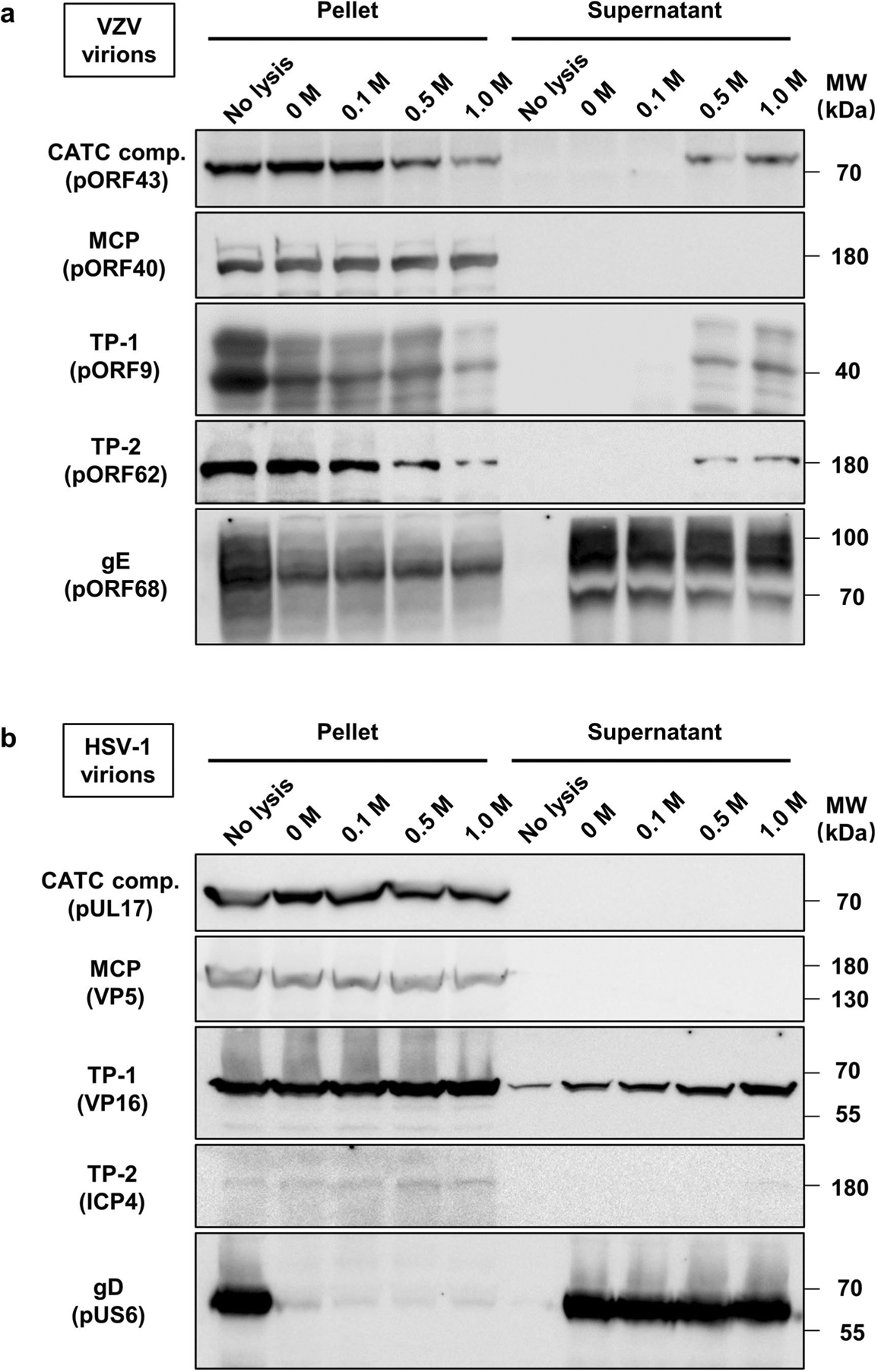
Purified virions of VZV (a) and HSV-1 (b) were not treated (no lysis) or treated with 1% Triton X-100 in the presence of 0, 0.1, 0.5, or 1M KCl. Viral lysates were then centrifuged through a 30% sucrose cushion. Pelleted (pellet) and released (supernatant) fractions were analyzed by SDS-PAGE and immunoblotting with antibodies targeting the CATC component (comp.) (VZV pORF43 and its HSV-1 counterpart, pUL17), MCP (VZV pORF40 and its HSV-1 counterpart, VP5), glycoproteins (VZV gE and HSV-1 gD), and other tegument proteins (TP; VZV pORF62 and pORF9, HSV-1 VP16 and ICP4). Markers of protein molecular mass (MW) are indicated in kDa on the right side of the panel. Experiments were performed two times independently with similar results.
