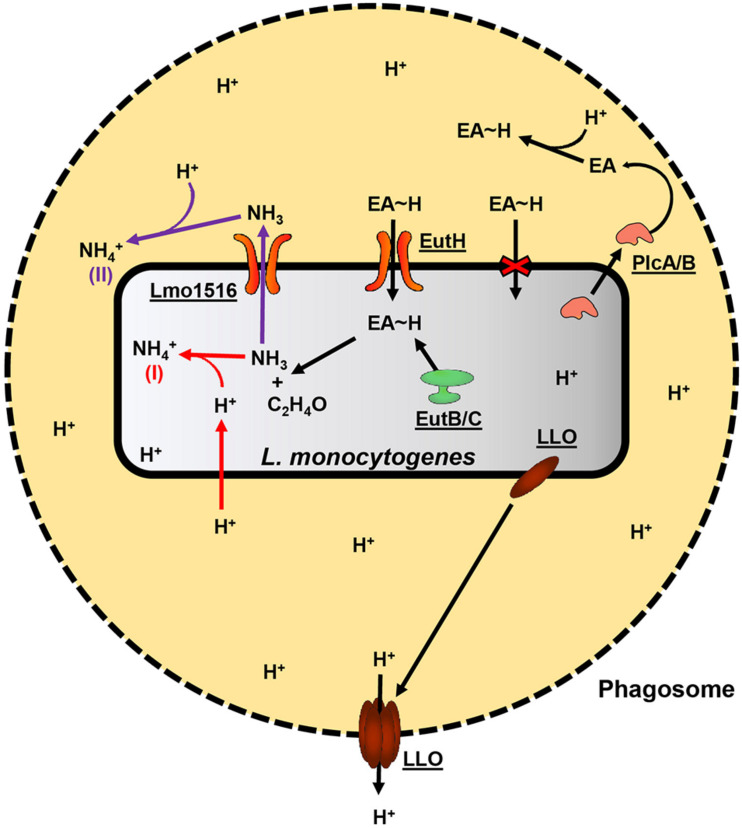
FIGURE 1.
Proposed model of EA oxidation and its contribution to the pathogenicity of L. monocytogenes. A black dashed perimeter indicates the membrane of the acidic phagosome. Free protons are denoted “H+,” and protein names or L. monocytogenes EGD-e locus tags are underlined. L. monocytogenes secretes LLO and PlcA/PlcB during its lifecycle within host-cell phagosomes. LLO will associate with the membrane of the phagosome and form pores, allowing protons to diffuse into the host cell cytosol, which, in turn, raises the internal pH of the phagosome. PlcA/PlcB may assist vesicle escape by degrading the host phospholipid membranes. In addition to the already present EA, degrading phosphatidylethanolamine found in host membranes would release additional EA into the vesicle environment. EA is quickly protonated (EA∼H) in the acidic conditions of the phagosome and is internalized by L. monocytogenes by the EutH transporter. The oxidation of the imported EA by the EutB/EutC complex yields acetaldehyde and ammonia. The acetaldehyde and ammonia may enter general carbon and nitrogen metabolism; however, the production of ammonia may contribute to the survival of L. monocytogenes during intracellular life in at least two mechanisms. In the first mechanism, denoted by a red “(I)” and red arrows, ammonia spontaneously reacts with a free proton (which may freely diffuse across L. monocytogenes cell membrane) within the Listeria cytosol and thereby increase intracellular pH by producing ammonium. In the second mechanism, denoted by a purple “(II)” and purple arrows, ammonia is excreted from L. monocytogenes and spontaneously reacts with protons outside the Listeria cell, yielding ammonium, and thereby providing a degree of localized protection from the acidic conditions of the phagosome.