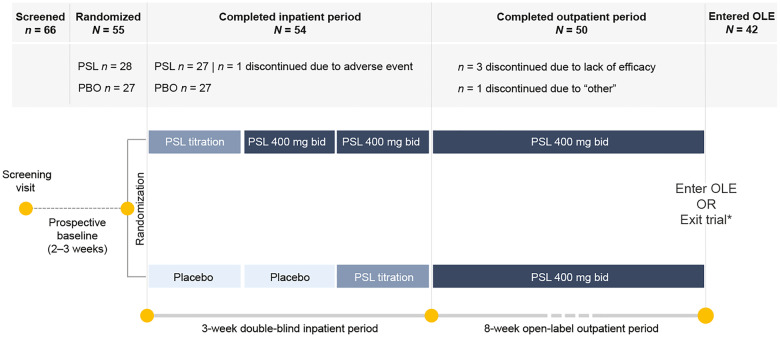
Figure 1.
Proof-of-concept trial design and patient disposition. Patients were randomized 1:1 to receive treatment with padsevonil (PSL) 400 mg bid or placebo. At the end of Week 2, patients in the placebo group transitioned to treatment with PSL 400 mg bid, and at the end of Week 3, all patients transitioned to open-label treatment with PSL 400 mg bid for 8 weeks. *At trial end, patients could either exit the trial and enter a safety follow-up period or continue treatment with PSL in an open-label extension (OLE).