Abstract
Background
Sudden cardiac death (SCD) is an uncommon but significant cause of death in the young. Citywide cardiac screening of school-aged children has been performed in Taipei since 1989. In this study, we investigate the efficacy of this screening method for identifying those at high risk of SCD.
Methods
This study analyzed the data from the results of cardiac screening for school-aged children in Taipei from 2003 to 2014. The cardiac screening included: Stage I, questionnaire surveys, simplified phonocardiography test and simplified electrocardiography (ECG) test; Stage II, physical examination and auscultation by a pediatric cardiologist for all children who had abnormal findings in stage I screening; Stage III, referral to a pediatric cardiologist for further examinations. Logistic regression and decision tree analyses were performed.
Results
A total of 566,447 students were screened, of whom 685 were identified as being at high risk of SCD. The most common causes of being at high risk of SCD included Wolff-Parkinson-White syndrome, long QT syndrome, cardiomyopathy and Marfan’s syndrome. Using logistic regression analysis, the simplified ECG test was identified as being the most effective tool (odds ratio = 16.4, p < 0.001) and past history as the second most crucial factor (odds ratio = 3.95, p < 0.001) for detecting a high risk of SCD. Decision tree analysis showed that serial studies with a past history and the simplified ECG test could accurately identify those at high risk of SCD.
Conclusions
Questionnaire survey and simplified electrocardiography test-based cardiovascular screening in school-aged children can identify those at high risk of SCD.
Keywords: Cardiac screening, Decision tree analysis, Logistic regression, School-age children, Sudden cardiac death
INTRODUCTION
Sudden death is defined as an unexpected death that occurs within a short period of time.1-3 The major cause of sudden death in the young is sudden cardiac death (SCD). It typically occurs within 1 hour of the onset of acute symptoms,4-8 and the causes include: (1) heart structure anomalies; (2) heart electrical disorders; and (3) acquired heart disease.9-12 Among these three types of disorders, the first two can be identified early by cardiac screening programs. Determining how to use appropriate screening methods for the early prevention and appropriate treatment of these groups at high risk of SCD is an important issue that needs to be urgently addressed.
Most previous research on SCD screening has focused on SCD in athletes, and only a few studies have focused on school-aged children.13 Taipei is the capital of Taiwan, and it has adequate medical resources and a high level of insight into its population’s health. The cardiac screening program was established by the Taipei City Government Education Bureau and has been conducted by the Cardiac Children’s Foundation Taiwan (CCFT) since 1989. The program has regularly conducted cardiac screening for students in the first grade of elementary school since 1999. Therefore, the large long-term database of the CCFT contains valuable information for investigating the feasibility and effectiveness of this cardiac screening program on preventing SCD. The purpose of the study was to explore whether the aforementioned cardiac screening program can detect a high risk of SCD.
METHODS
Cardiac screening for school-aged children by the CCFT
Data were obtained from the school-aged children’s cardiac screening program database provided by the CCFT. Variables containing personal privacy information were deleted. All first grade elementary school-aged children screened between September 1, 2003, and June 30, 2014 were included. A total of 566,447 children participated in the first stage of cardiac screening. In the first stage, the examination included questionnaires pertaining to self-recognized symptoms, past history and family history, as well as a simplified electrocardiogram (ECG) test that included lead I, aVF, VI and V6, and a simplified phonocardiograph (PCG) test (Figure 1).
Figure 1.
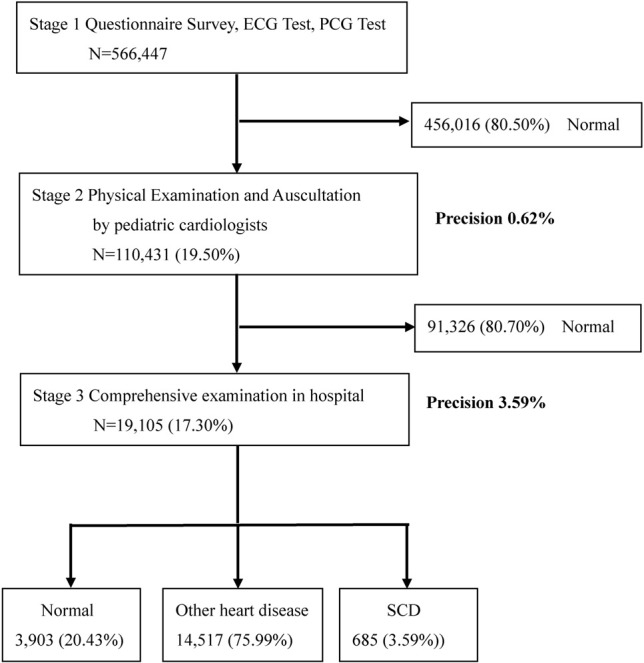
Cardiac screening flow chart. Through stage 1 cardiac screening, we found 110,431 children with suspected heart disease; through the stage 2 of cardiac screening, we referred 19,105 children to hospital for further examination. In stage 3, pediatric cardiologists in the pediatric cardiology center arranged comprehensive examinations such as chest X ray, electrocardiogram, echocardiogram and treadmill to confirm the diagnosis of heart disease (high risk SCD N = 685, other heart disease N = 14,517). The precision increased from 0.62% in stage 2 screening to 3.59% in stage 3 screening. ECG, electrocardiogram; PCG, phonocardiograph; SCD, sudden cardiac death.
The questionnaire survey about self-recognized symptoms included: palpitations, sudden onset of fainting or syncope, exertional chest pain (excluding growing pain), recent pain in two or more joints of the limbs (excluding trauma), exertional dyspnea, pale appearance, cyanosis, involuntary movements, erythema marginatum on the trunk or proximal limbs, subcutaneous nodules on the elbows, ankles, or joints, and persistent fever for more than 5 days associated with skin rashes and fingertip desquamation. The questionnaire survey of past history included: previously diagnosed cardiomyopathy, Kawasaki disease, acute rheumatic fever, rheumatic heart disease, congenital heart disease, arrhythmia, hyperlipidemia, valvular heart disease, other heart disease and mitral valve prolapse. The survey about family history was related to cardiovascular disease.
The completed data were sent to a pediatric cardiologist for interpretation. Any children with at least one abnormal item listed above received a second-stage evaluation. In the second stage, the children received a physical examination and also past history taking if necessary by a pediatric cardiologist at their school. The pediatric cardiologist identified the children as being: (1) healthy or (2) needing referral to a hospital for further examination. In the third stage, pediatric cardiologists at the pediatric cardiology center arranged further studies such as chest X ray, electrocardiogram, echocardiogram and Treadmill or 24-hour Holter examination to confirm the diagnosis of heart disease, and the results of these examinations in the hospital were recorded by the CCFT. Among the 566,447 children who participated in the first stage of cardiac screening, 456,016 (80.5%) were considered to be healthy, and 110,431 (19.5%) were suspected to be at high risk of SCD or to have cardiovascular disease. The data used in this study included those of all suspected students (N = 110,431).
A high risk of sudden cardiac diseases was defined as follows: (1) structural heart anomalies including dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, pulmonary hypertension, Eisenmenger’s syndrome, Marfan’s syndrome, and congenital complex heart disease; (2) heart electrical disorders including atrial fibrillation, atrial flutter, complete atrioventricular block, long QT syndrome, ventricular fibrillation, ventricular tachycardia, Wolff-Parkinson-White syndrome, Brugada syndrome, supraventricular tachycardia, and arrhythmogenic right ventricular dysplasia.
Regression model
We used a logistic regression model to detect important factors to screen for a high risk of SCD. We were able to compare the relative effects for the independent variables because all of the variables were zero-one binary variables, where 1 represents "yes" and 0 represents "no". The models for SCD were as follows:
Model 1: high risk of SCD model
SCD = γ0 + γ1 SEX + γ2 SRS + γ3 PH + γ4 FH + γ5 ECG + γ6 PCG
SCD: high risk of sudden cardiac death
SEX: gender (male = 1, female = 0)
SRS: self-recognized symptoms
PH: past history
FH: family history
ECG: simplified ECG test
PCG: simplified PCG test
In disease association studies, a more stringent cut-off of 0.01 is recommended.14 Therefore, to reduce the false detection rate, an adjusted P threshold/cutoff of 0.01 was used in this study.
We also used decision tree analysis to provide tree-like classification of the sample. The chi-square automatic interaction detector is an adopted algorithm used for decision tree analysis. The decision tree can help to understand the proportion of those at high risk of SCD and healthy cases under a variety of different situations. p < 0.01 was considered to be statistically significant.
RESULTS
Descriptive statistics
Table 1 shows the descriptive statistics for the suspected 110,431 cases. A positive past history was recorded in 18.48% of the children, and 35.43% of the children reported self-recognized symptoms. A family history was recorded in 27.85% of the children, but many parents could not be sure whether or not they had a positive family history of cardiac disease. There were 6,711 (6.08%) missing data in the item of family history. An abnormal simplified PCG test result was recorded in 6.29% of the students, and an abnormal simplified ECG test result was identified in 35.70% of the children. In total, 95,229 (86.23%) children were considered to be healthy (no heart disease), 685 (0.62%) children were identified as being at high risk of SCD, and 14,517 (13.15%) were classified as having other types of heart disease.
Table 1. Descriptive statistics of 110,431 students receiving pediatric cardiology clinics evaluation.
| N | % | |
| Gender | ||
| Male | 51,581 | 46.71% |
| Self-recognized symptoms | ||
| Yes | 39,129 | 35.43% |
| Past history | ||
| Yes | 20,404 | 18.48% |
| Family history | ||
| Yes | 30,750 | 27.85% |
| Missing | 6,711 | 6.08% |
| Simplified ECG test | ||
| Abnormal | 39,427 | 35.70% |
| Simplified PCG test | ||
| Abnormal | 6,949 | 6.29% |
| Total/N | 110,431 | 100.00% |
In this study we used precision analysis which increased from 0.62% in stage II screening to 3.59% in stage III screening (Figure 1). The precision analysis displayed the process of layer-by-layer accuracy improvement through the staged screening.
Table 2 shows the disease category and number of various diseases causing a high risk of SCD. Fourteen diseases were identified in the 685 students defined as being at high risk of SCD, among which Wolff-Parkinson-White syndrome was the most common (N = 523), followed by long QT syndrome (N = 40), and Marfan’s syndrome (N = 29). There were 44 cases of cardiomyopathy diseases (cardiomyopathy, dilated cardiomyopathy, and hypertrophic cardiomyopathy), and congenital heart disease accounted for 17 cases (Eisenmenger’s syndrome and congenital complex heart disease).
Table 2. The disease categories of positive screening for high risk of SCD in school-aged children in Taipei city from 2003 to 2014.
| Sudden arrhythmic death syndrome (SADS) | N |
| Wolfe-Parkinson-White syndrome | 523 |
| Long QT syndrome | 40 |
| Marfan’s syndrome | 29 |
| Cardiomyopathy | 26 |
| Congenital complex heart | 16 |
| Hypertrophic cardiomyopathy | 15 |
| Atrioventricular block (3rd degree) | 13 |
| Ventricular tachycardia | 9 |
| Pulmonary hypertension | 6 |
| Dilated cardiomyopathy | 3 |
| Atrial flutter | 2 |
| Atrial fibrillation | 1 |
| Ventricular fibrillation | 1 |
| Eisenmenger’s syndrome | 1 |
| Total | 685 |
SCD, sudden cardiac death.
Logistic regression
Table 3 shows the models of a high risk of SCD. The simplified ECG test was the most effective tool for detecting a high risk of SCD (β = 2.800, p < 0.001, odds ratio = 16.442). Past history was the second most significant factor for detecting a high risk of SCD (β = 1.374, p < 0.001, odds ratio = 3.952).
Table 3. Logistic regression analysis to identify high risk of SCD.
| Variable | Dependent variable: sudden cardiac death | ||||
| β | Std. error | Odds ratio | 95% confidence interval | ||
| Lower | Upper | ||||
| Constant | -7.200 | 0.131 | |||
| Gender | -0.070 | 0.080 | 0.933 | 0.80 | 1.09 |
| Self-recognized symptoms | 0.210 | 0.100 | 1.234 | 1.01 | 1.50 |
| Past history | 1.374** | 0.090 | 3.952 | 3.31 | 4.71 |
| Family history | -0.075 | 0.091 | 0.928 | 0.78 | 1.11 |
| Simplified ECG test | 2.800** | 0.118 | 16.442 | 13.04 | 20.74 |
| Simplified PCG test | 0.297 | 0.139 | 1.346 | 1.03 | 1.77 |
| -2 log-likelihood | 6,471.222 | ||||
| Nagelkerke R2 | 0.167 |
Note: β is the coefficient of logistic regression. Std. error is the standard error of the estimated coefficient.
** p < 0.01 same finding based on bootstrap and maximum likelihood estimation.
SCD, sudden cardiac death.
Table 4 shows the association of the simplified ECG test and high risk of SCD. For the children at high risk of SCD, 86% had abnormal ECG findings. This was significantly higher than the percentage categorized as not being at high risk of SCD.
Table 4. Cross table of the simplified ECG test and the high risk of SCD.
| ECG normal | ECG abnormal | Total | |
| Non-high risk of SCD | 70,908 (64.6%) | 38,838 (35.4%) | 109,746 (100.0%) |
| High risk of SCD | 96 (14.0%) | 589 (86.0%) | 685 (100.0%) |
| Total | 71,004 (64.3%) | 39,427 (35.7%) | 110,431 (100.0%) |
ECG, electrocardiogram; SCD, sudden cardiac death.
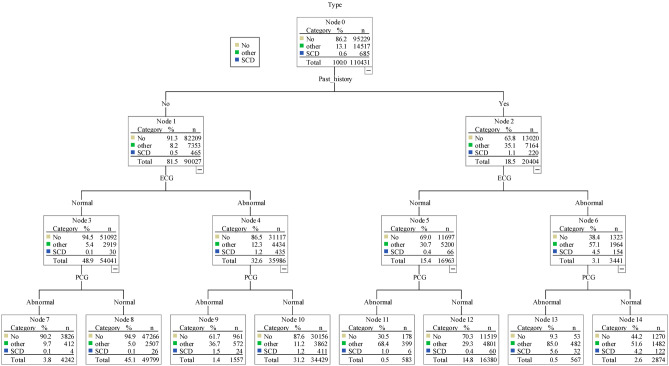
Decision tree method for classification
Figure 2 shows the decision tree classification for a high risk of SCD, other types of heart disease, and a non-high risk of SCD. In the 685 children categorized as being at high risk of SCD, 465 had no past history. Among these 465 children, 435 had abnormal ECG findings and were identified as being at high risk of SCD (odds ratio = 22.029, β = 3.092, p < 0.001). Similarly, for the children who had a positive past history, the incidence of being at high risk of SCD in the abnormal ECG group (N = 154) was higher than that in the normal ECG group (N = 66, odds ratio = 11.995, β = 2.484, p < 0.001).
Figure 2.
Decision tree analysis of sudden cardiac death. The samples in this decision three analysis included 110,431 children. Three types of outcome variable were identified in the analysis including high risk SCD (i.e., SCD), other heart disease (i.e., other), and healthy cases (i.e., No). A total of 15,202 children referred to the hospital in the third stage were diagnosed with heart problems. These children with heart disease included 685 high risk SCD cases and 14,517 other heart disease cases. The analysis of decision tree shows that past history and simplified ECG test were two most import variables to classify these three types. In the beginning, there are 0.6% cases who were identified as high risk SCD. In the right half, for children who had past history (i.e., “Yes”), the SCD percentage increased to 1.1% (from 0.6% to 1.1%). Among those with past history “Yes”, the SCD percentage increase to 4.5% (from 1.1% to 4.5%) if their simplified ECG test are abnormal. Furthermore, among those with past history and simplified ECG test are abnormal (N = 3341), the SCD percentage increase to 5.6% (from 4.5% to 5.6%) if the simplified PCG test is abnormal. In the left half, for children who had “No” past history (N = 90027). The percentage of high risk SCD is about 0.5% (N = 465). However, if simplified ECG test is abnormal, the percentage of high risk SCD increase to 1.2%. If the both simplified ECG test and simplified PCG test are abnormal, the high risk SCD increase further to 1.5% (N = 24). The children without past history and normal initial simplified ECG results, simplified PCG test has little benefit in identifying high risk of SCD (0.1% to 0.1%). For the 26 students without past history and normal initial simplified ECG and simplified PCG results, they were referred for further examination because the pediatric cardiologist judged the PCG result was abnormal in 4, the ECG result was abnormal in 19, and 7 students had abnormal physical examination. The diagnoses of these 26 students included hypertrophy cardiomyopthy 1 case, WPW syndrome 11 cases, and long QT syndrome 14 cases. Note: “Other” indicates other heart disease. “No” indicates non-high risk of SCD. “SCD” indicates high risk of SCD. The adjusted p < 0.01 for all comparisons. ECG, electrocardiogram; PCG, phonocardiograph; SCD, sudden cardiac death.
DISCUSSION
Using questionnaires, simplified ECG test and simplified PCG test-based cardiac screening, 685 students at high risk of SCD were identified from 566,447 school-aged children. The simplified ECG test and positive past history were the most important variables for predicting those at high risk of SCD. Sudden unexpected death is a significant but uncommon cause of death in the young. Its dramatic presentation and effects on the family and community make it a newsworthy and important public health issue. Wu et al. (2010)15 reported that the incidence rate of SCD in children under the age of 18 in Taiwan is about 2.7 per 100,000 people, which is similar to the incidence rate in Western countries (about 0.6-6.2 per 100,000 people). Because sudden death without previous warning signs is the first symptom in nearly half of the patients, screening these high risk patients is very important. The decision to screen for a disease is generally based on the following conditions:16 (1) the disease is an important public issue; (2) the screening has an appropriate target; (3) the screening is safe and economical; and (4) the disease can be appropriately treated. We suggest that as SCD is an important disease with serious complications, it meets these criteria and is suitable for widespread screening.
Several noninvasive and economical screening methods have been suggested to identify patients at high risk of SCD. Because of cost benefit considerations in Western countries, most of the screening targets for SCD are athletes,17-27 who have a risk of SCD 3-4 times higher than the general population. The United States and Europe have adopted different programs for SCD screening in athletes. In the United States, past history and physical examinations are used as screening tools; in contrast, in Europe, in addition to past history and physical examinations, ECG has also been adopted as a screening tool.28-30 There is strong debate about the cost effectiveness of ECG as a screening tool in these two groups. The American group suggest that due to the low incidence of sudden death as well as a high false positive rate,31 there is no need to add ECG to the screening program.32 Conversely, the European group suggest that adding ECG screening can effectively reduce the incidence of SCD.32 In the present study, we showed that a simplified ECG test was the most significant variable for the detection of high risk SCD in logistic regression analysis. Using decision tree analysis, we showed that a past history of heart disease alone could not effectively determine those at high risk of SCD. Using past history alone, we would have missed 465 patients who would otherwise be categorized as being at high risk of SCD after adding ECG. Regarding the subgroup without a past history, the incidence of being at high risk of SCD in those with an abnormal ECG was 12 times (1.2%) higher than in those with anormal ECG (0.1%). Consistent with the European group, this demonstrates that ECG is indeed an important screening tool for SCD.32
In contrast to Western countries, Japan uses ECG screening in all school-aged children at the first grade of elementary school, junior and senior high school.33,34 Despite the great financial cost of population screening, considering the life-time savings and great impact on families and society when a young person dies of SCD, they still suggest that it is cost effective for young populations. In our country, because of the low birth rate, SCD in the young has an even greater impact on society. Therefore, we implemented population cardiac screening in school-aged children for SCD.
Through this cardiac screening program, 110,431 suspected cases were identified first from 566,477 school-aged children. Furthermore, 685 were identified as being at high risk of SCD. This means that through appropriate screening, we could narrow down those at high risk of SCD from a large population to fewer than 700 children. More importantly, in 110,431 suspected cases, 90,027 had no past history. Among these 90,027 patients, 7,353 were diagnosed as having other heart diseases and 465 were diagnosed to be at high risk of SCD after screening. With appropriate interventions and follow-up, the incidence of SCD in young children can be greatly reduced.
In contrast to previous reports, in the present study, self-recognized symptoms and family history of SCD had no effect on identifying high risk cases of SCD. There are several possible reasons for this finding. First, many children who are just entering elementary school are too young to express self-recognized symptoms. In addition, previous studies have also shown that more than half of the patients at high risk of SCD had no related prodromal symptoms. With respect to family history, 6,711 questionnaires had missing data, and the reported cardiac-related family history was sometimes not relevant. Many parents reported their metabolic syndrome-related cardiovascular diseases as myocardial infarction and hypertension, which may not be related to young SCD. Lastly, it is also possible that parents with an SCD-related family history did not report it due to reasons of personal privacy.
Study limitations
Some limitations of this study should be considered when interpreting the results. More than 500,000 students were involved in heart screening. The database has records of only 110,431 suspected children because healthy children do not need to be tracked for further investigation. However, the other 456,016 children may still have had a small risk of having SCD.
The incidence rate of SCD in elementary school-aged children is around 2.7/100000 in our countrywide National Health database. In the present study, we were concerned only about those at high risk of SCD rather than the actual incidence of SCD as we did not track the long-term outcomes of these high risk children. Besides, we cannot link to the death records of the National Health Bureau because of personal information protection policy and because we did not obtain permission from the patients. More research is needed to explore whether such screening programs can truly reduce the incidence of SCD in society. The third limitation concerns the school testing environment and the simplified ECG test per se, as it recorded only four and not 12 leads. Nonetheless, our previous study confirmed its efficacy to identify cardiac rhythm abnormalities in screening school-aged children.16
CONCLUSION
The questionnaire survey and simplified electrocardiography test-based cardiovascular screening in school-aged children can identify high risk population of SCD.
FUNDING
This work was supported by the National Science Council in Taiwan [106-2314-B-002-008-, 107-2314-B-002-008-, 108-2314-B-002-008-].
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Asif IM, Harmon KG. Incidence and etiology of sudden cardiac death: new updates for athletic departments. Sports Health. 2017;9:268–279. doi: 10.1177/1941738117694153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mag S, Lariccia V, Maiolino M, et al. Sudden cardiac death: focus on the genetics of channelopathies and cardiomyopathies. J Biomed Sci. 2017;24:56. doi: 10.1186/s12929-017-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barletta V, Fabiani I, Lorenzo C, et al. Sudden cardiac death: a review focused on cardiovascular imaging. J Cardiovasc Echogr. 2014;24:41–51. doi: 10.4103/2211-4122.135611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison SR. Sudden cardiac death in adolescents. Primary Care: Clinics in Office Practice. 2015;42:57–76. doi: 10.1016/j.pop.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Leslie LK, Cohen JT, Newburger JW, et al. Costs and benefits of targeted screening for causes of sudden cardiac death in children and adolescents. Circulation. 2012;125:2621–2629. doi: 10.1161/CIRCULATIONAHA.111.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley MB, Larsen MK. Pericardial cyst: cause of sudden cardiac death? J Forensic Sci. 2019;64:295–297. doi: 10.1111/1556-4029.13826. [DOI] [PubMed] [Google Scholar]
- 7.Sara JD, Eleid MF, Gulati R, et al. Sudden cardiac death from the perspective of coronary artery disease. Mayo Clin Proc. 2014;89:1685–1698. doi: 10.1016/j.mayocp.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Mampilly NT, Ravikumar G, Alben B, et al. Sudden death in a child:the mystery behind the cardiac mass. J Clin Diagnostic Res. 2019;13:8–10. [Google Scholar]
- 9.Morris VB, Keelan T, Leen E, et al. Sudden cardiac death in the young: a 1-year post-mortem analysis in the Republic of Ireland. Ir J Med Sci. 2009;178:257–261. doi: 10.1007/s11845-009-0294-8. [DOI] [PubMed] [Google Scholar]
- 10.Wever EFD, de Medina EOR. Sudden death in patients without structural heart disease. J Am Coll Cardiol. 2004;43:1137–1144. doi: 10.1016/j.jacc.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 11.Hookana E, Junttila MJ, Puurunen VP, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–1575. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Thiene G, Pennelli N, Rossi L. Cardiac conduction system abnormalities as a possible cause of sudden death in young athletes. Hum Pathol. 1983;14:704–709. doi: 10.1016/s0046-8177(83)80143-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitani Y, Ohta K, Yodoya N, et al. Public access defibrillation improved the outcome after out-of-hospital cardiac arrest in school-age children: a nationwide, population-based, Utstein registry study in Japan. Europace. 2013;15:1259–1266. doi: 10.1093/europace/eut053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari M, Ansari-Pour N. Why, when and how to adjust your P values? Cell Journal (Yakhetech) 2019;20:604–607. doi: 10.22074/cellj.2019.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MH. Sudden death in pediatric populations. Korean Circ J. 2010;40:253–257. doi: 10.4070/kcj.2010.40.6.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu SN. Heart disease screen of children’s sudden cardiac death. Arrhythmia News. 2013;12:2–3. [Google Scholar]
- 17.Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644–648. doi: 10.1136/bjsm.2008.054718. [DOI] [PubMed] [Google Scholar]
- 18.Corrado D, Basso C, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden cardiac death? J Cardiovasc Med. 2006;7:228–233. doi: 10.2459/01.JCM.0000219313.89633.45. [DOI] [PubMed] [Google Scholar]
- 19.Corrado D, Basso C, Schiavon M, et al. Pre-participation screening of young competitive athletes for prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1981–1989. doi: 10.1016/j.jacc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Chandra N, Bastiaenen R, Papadakis M, Sharma S. Sudden cardiac death in young athletes: practical challenges and diagnostic dilemmas. J Am Coll Cardiol. 2013;61:1027–1040. doi: 10.1016/j.jacc.2012.08.1032. [DOI] [PubMed] [Google Scholar]
- 21.Cross BJ, Estes NAM, III, Link MS. Sudden cardiac death in young athletes and nonathletes. Curr Opin Crit Care. 2011;17:328–334. doi: 10.1097/MCC.0b013e328348bf84. [DOI] [PubMed] [Google Scholar]
- 22.Halkin A, Steinvil A, Rosso R, et al. Preventing sudden death of athletes with electrocardiographic screening: what is the absolute benefit and how much will it cost? J Am Coll Cardiol. 2012;60:2271–2276. doi: 10.1016/j.jacc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Harmon KG. Routine ECG screening of young athletes: can this strategy ever be cost effective? J Am Coll Cardiol. 2016;68:712–714. doi: 10.1016/j.jacc.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 24.McCaffrey FM, Braden LDS, Strong WB. Sudden cardiac death in young athletes: a review. Am J Dis Child. 1991;145:177–183. doi: 10.1001/archpedi.1991.02160020069020. [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ, Gohman TE, Aeppli D. Prevalence of sudden cardiac death during competitive sports activities in Minnesota high school athletes. J Am Coll Cardiol. 1998;32:1881–1884. doi: 10.1016/s0735-1097(98)00491-4. [DOI] [PubMed] [Google Scholar]
- 26.Roberts WO, Asplund CA, O'Connor FG, et al. Cardiac preparticipation screening for the young athlete: why the routine use of ECG is not necessary. J Electrocardiol. 2015;48:311–315. doi: 10.1016/j.jelectrocard.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Estes NAM, III, Vetter VL, Corrado D. Cardiac screening before participation in sports. N Engl J Med. 2013;369:2049–2053. doi: 10.1056/NEJMclde1311642. [DOI] [PubMed] [Google Scholar]
- 28.Harmon KG, Zigman M, Drezner JA. The effectiveness of screening history, physical exam, and ECG to detect potentially lethal cardiac disorders in athletes: a systematic review/meta-analysis. J Electrocardiol. 2015;48:329–338. doi: 10.1016/j.jelectrocard.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 30.Schmehil C, Malhotra D, Patel DR. Cardiac screening to prevent sudden death in young athletes. Transl Pediatr. 2017;6:199–206. doi: 10.21037/tp.2017.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetter VL, Dugan N, Guo R, et al. A pilot study of the feasibility of heart screening for sudden cardiac arrest in healthy children. Am Heart J. 2011;161:1000–1006. doi: 10.1016/j.ahj.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Schoenbaum M, Denchev P, Vitiello B, Kaltman JR. Economic evaluation of strategies to reduce sudden cardiac death in young athletes. Pediatrics. 2012;130:e380–e389. doi: 10.1542/peds.2011-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niwa K, Warita N, Sunami Y, et al. Prevalence of arrhythmias and conduction disturbances in large population-based samples of children. Cardiol Young. 2004;14:68–74. doi: 10.1017/s104795110400112x. [DOI] [PubMed] [Google Scholar]
- 34.Tasaki H, Hamasaki Y, Ichimaru T. Mass screening for heart disease of school children in Saga city: 7-year follow up study. Jpn Circ J. 1987;51:1415–1420. doi: 10.1253/jcj.51.1415. [DOI] [PubMed] [Google Scholar]