figs4.
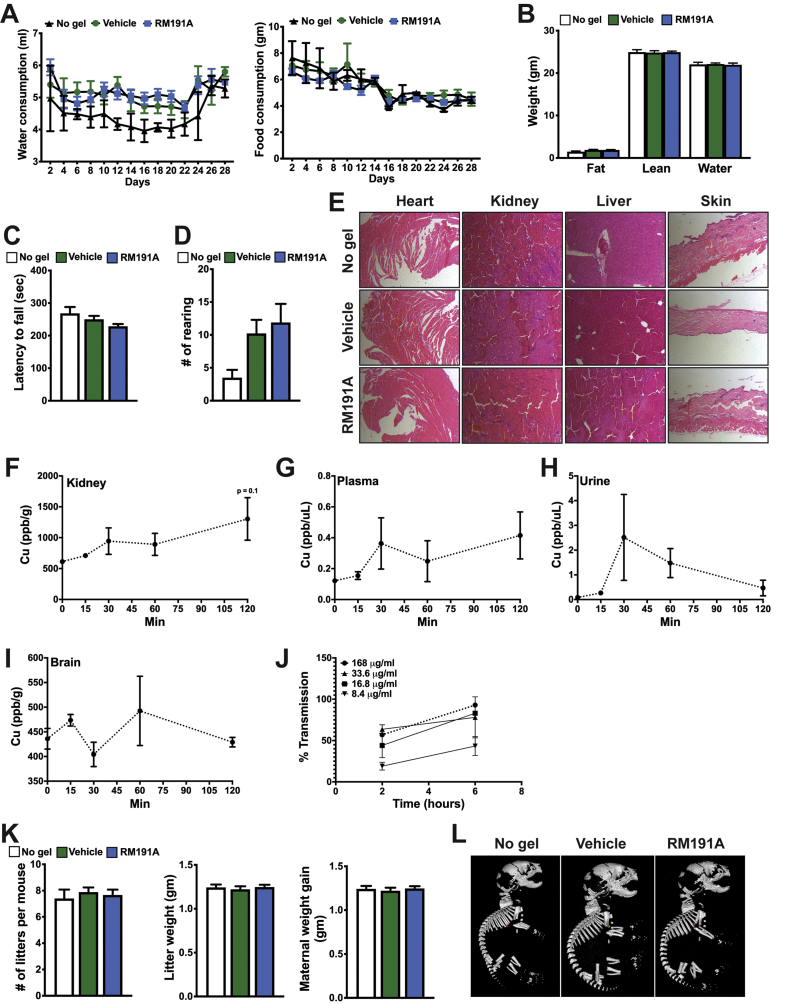
Figure S4: RM191A is nontoxic, non-teratogenic and readily bioavailable in mice
(A) Water and food consumption, (B) EchoMRI, (C) rotarod performance and (D) rearing behaviour of mice untreated and treated with topical doses of RM191A (0.19 mL/kg) or Vehicle for 29 days (n = 9). (E) H&E staining of heart, kidney, liver and skin from mice untreated and treated with topical doses of RM191A or Vehicle for 29 days. Copper levels in (F) kidney, (G) plasma, (H) urine and (I) brain of mice treated with RM191A (50 μL, 1.9 mL/kg body weight), harvested over different time points and measured using ICP-MS (n = 4 per time point). (J) Percentage transmission of RM191A over time in the in vitro blood-brain-barrier model (n = 2). (K) Quantification of the number of litters produced per pregnant mouse, average weight of litter and maternal weight gain (difference in the body weight between gestational day 0 and 18) in the teratogenicity study (n = 9). Pregnant females were left untreated or treated daily topically with RM191A (50 μL of 2.1 mg/mL)/Vehicle from GD 4 until GD18. (L) Representative μ-CT images of P0 pups from untreated, RM191A-treated and Vehicle-treated mice.