Fig. 1.
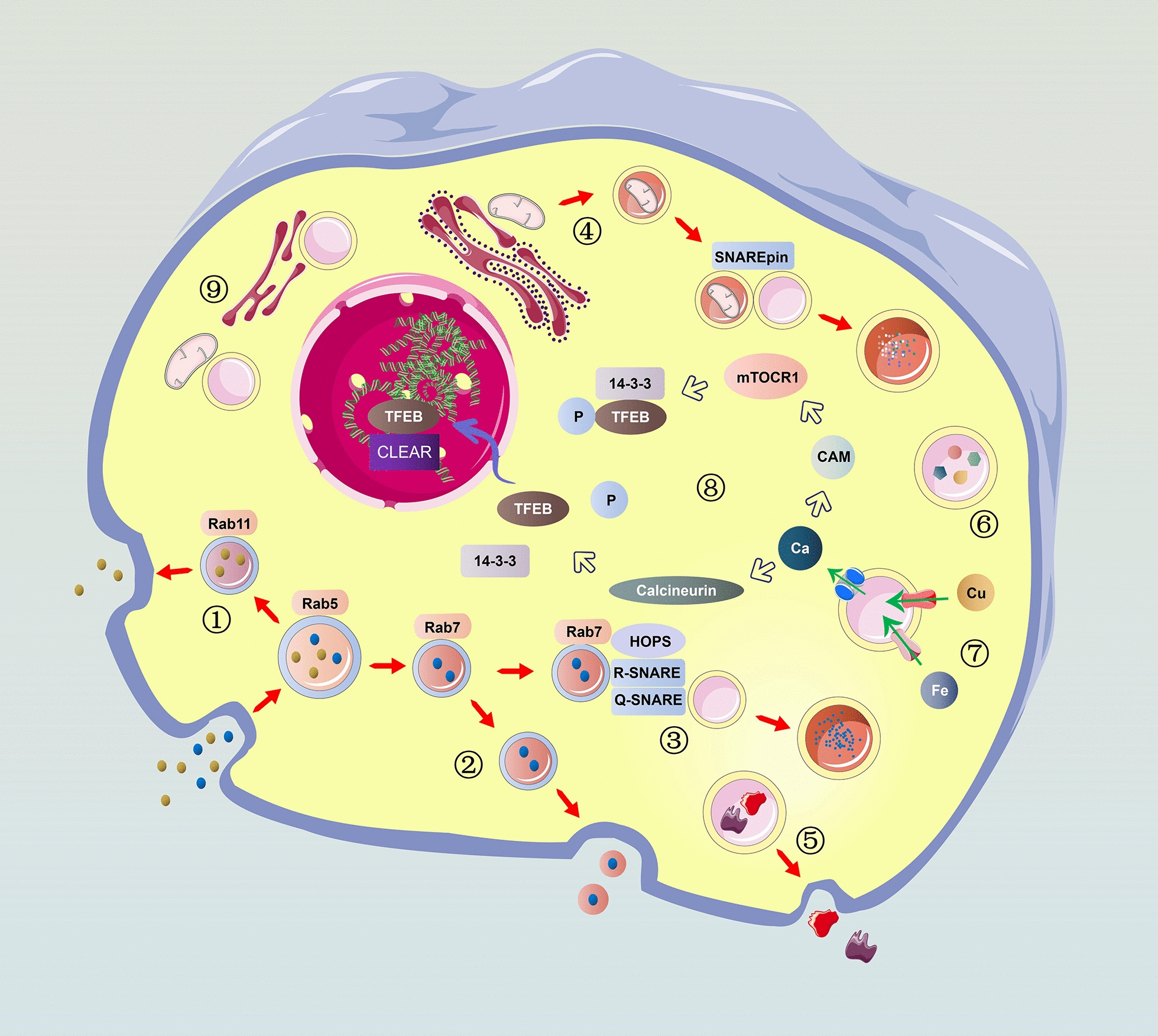
Lysosomes play a crucial role in intracellular transport. Vesicles formed by endocytosis and phagocytosis deliver cargo to Rab5-positive early endosomes. (1) Materials can be recycled to the plasma membrane by Rab11-positive recycling endosomes. (2) The remaining contents will be sequestered in Rab7-positive late endosomes, which can fuse with the plasma membrane to form exosomes. (3) Late endosomes can also fuse with lysosomes to degrade their cargo. During this process, Rab7 promotes the assembly of HOPS, which mediates lysosomal tethering with endosomes by pairing an R-SNARE on a lysosome (VAMP7 or VAMP8) with three Q-SNAREs on an endosome (syntaxin-7, VTI1b, syntaxin-8). (4) Lysosomal fusion with autophagosomes also requires SNAREs, including VAMP8, syntaxin-17 and SNAP29. (5) Lysosomes can also fuse with the plasma membrane to mediate membrane repair or discharge contents outside the cell, such as cathepsins or immune factors. (6) Lysosomes are the pools of metabolites in cells, including amino acids, sugars, lipids and nucleotides. (7) Metal ions are also stored within lysosomes. The storage of iron or copper can prevent their harmful accumulation in cells. (8) Lysosomal calcium channels, such as TRPMLs, can lead to the release of lysosomal calcium and activate mTORC1, which can phosphorylate TFEB and prevent TFEB nuclear translocation. TRPML1-mediated lysosomal calcium release can also dephosphorylate TFEB and promote its nuclear translocation and regulate lysosome biogenesis, autophagy, and lipid metabolism. (9) Lysosomes can form physical contacts with the ER, mediating the rapid transport of lipids, or with mitochondria, promoting mitochondrial fission or regulating the tricarboxylic acid cycle
