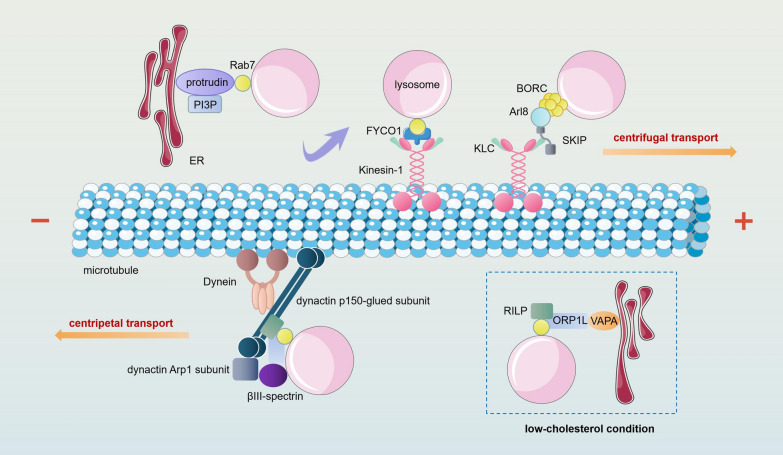
Fig. 3.
Movement and spatial distribution of lysosomes. Lysosomes can move towards the plus or minus ends of microtubules. The movement towards the plus end of microtubules is mediated by kinesins, of which the best characterized is kinesin-1, which is composed of two heavy chains (KHC) and two light chains (KLC). BORC can associate with lysosomes and recruit Arl8. SKIP binds to Arl8 through an N-terminal RUN domain and interacts with KLC through a WD motif in an unstructured region. Moreover, the ER-anchored protein protrudin binds simultaneously to Rab7 and PI3P to bridge the ER and lysosome. Protrudin then transfers lysosomes to the Rab7 effector FYCO1 and kinesin-1. The movement towards the minus end of microtubules is mediated by dynein. Lysosomal transport mediated by dynein is dependent on dynactin. The recruitment of dynein-dynactin to lysosomes is dependent on Rab7. RILP can link Rab7 to the dynactin p150-glued subunit. ORP1L forms a tripartite complex with Rab7 and RILP, promoting the association of βIII-spectrin with the dynactin Arp1 subunit. The interaction of ORP1L with Rab7–RILP and βIII-spectrin can activate dynein. The ability of ORP1L to engage is dependent on cholesterol levels. Under low-cholesterol conditions, ORP1L will bind VAPA, resulting in the dissociation of dynein-dynactin from lysosomes