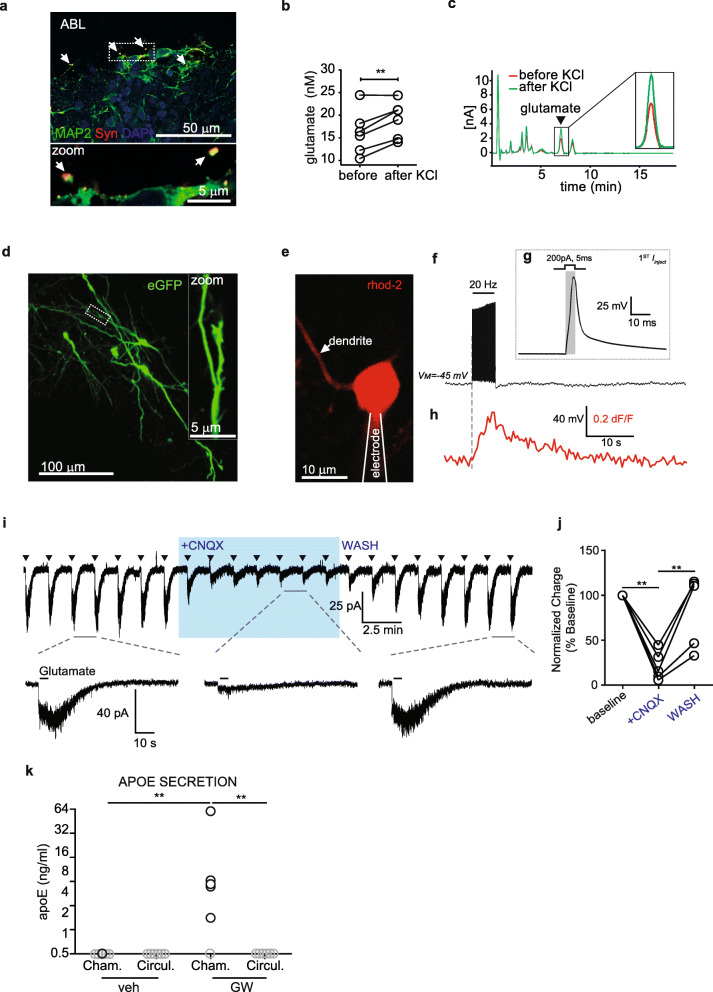
Fig. 2.
Depolarization- and glutamate-driven activity in abluminal cells indicates a neuronal phenotype and apoE secretion in the tissue chamber indicates astrocyte function and endothelial barrier formation. a Immunostaining against MAP 2 and synapsin-I (Syn) confirmed the presence of synapses in iPSC-derived neurons cultured in arterial NVU. b Glutamate release measured by HPLC showing increase after KCl treatment. c Example HPLC curves. d Two-photon Z-projection image of iPSC cells expressing eGFP. Dotted box displays region of zoomed inset, highlighting dendritic morphology and synaptic structure. e Example image of a whole-cell patch clamped iPSC-derived neuron dialyzed with the red Ca2+-indicator Rhod-2. Proximal dendrites were imaged for depolarization-induced Ca2+-entry. f Representative current-clamp trace from patched cell in ‘e’ during 20 Hz spike train stimulation. g Single current injection (200 pA, 5 ms) example from ‘f’ showing change in membrane potential. h Time-correlated Rhod-2 signal from trace ‘e’ showing depolarization-induced Ca2+-increase. i (Top trace) Full-length voltage-clamp recording showing glutamate puff-evoked (triangles) AMPAR currents that were amenable to block by CNQX (10 μM) and recovered in washout. (Bottom trace) Example AMPAR currents before, during, and after CNQX application. j Quantitative summary of normalized charge for glutamate inward currents in the presence of CNQX (n = 5, **P < 0.01). k Astrocyte and endothelium barrier functions were confirmed by treating tissues with 1 μM LXR agonist GW3965 for 96 h and measuring the levels of astrocyte-derived apoE secreted into the tissue chamber and circulation media. Values below the detection of the ELISA are plotted in gray. Points in graphed data represent individual bioengineered vessels, bars represent mean, error bars represent ±SEM and analysed by one way ANOVA **P < 0.01