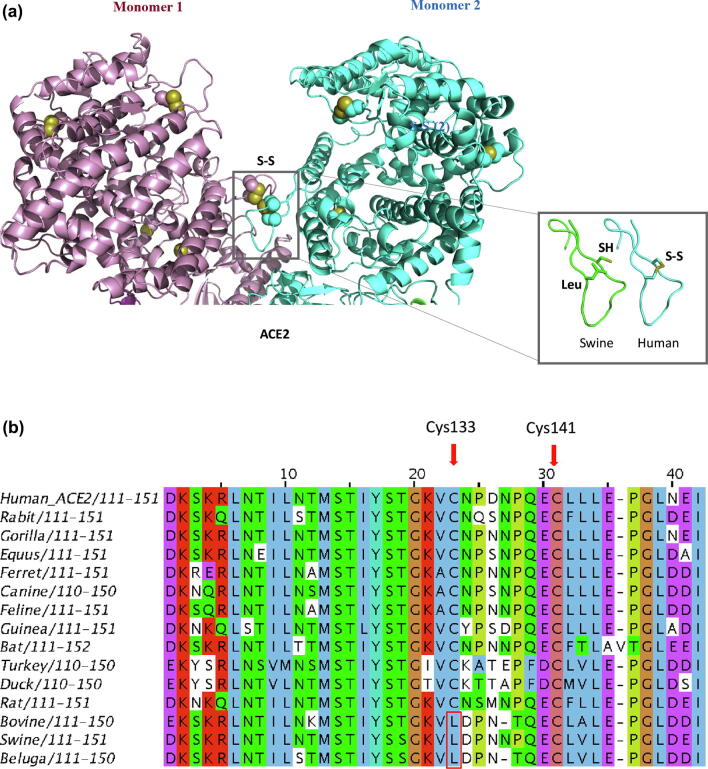
Fig. 2.
a: Ribbon representation of the extracellular domain of the homodimeric human ACE2 receptor (PDB code: 6m17; Ref. [8]). Sidechain of cysteine amino acids are shown in spacefill. The ACE2 protein has three disulfides. One disulfide participates in the interaction between monomers. A model of the loop with the mutated amino acid is shown on the right of the figure. The absence of disulfide may influence the conformational stability of the loop, with implications for dimer formation. b: Alignment of host receptor Angiotensin Converting Enzyme II (ACE2) sequences from different organisms. Cysteine residue (Cys133) replaced with leucine in cattle, swine and beluga is shown in the red box. Disulfide between Cys133 and Cys141 has been predicted for redox potential and denoted by red arrows. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)