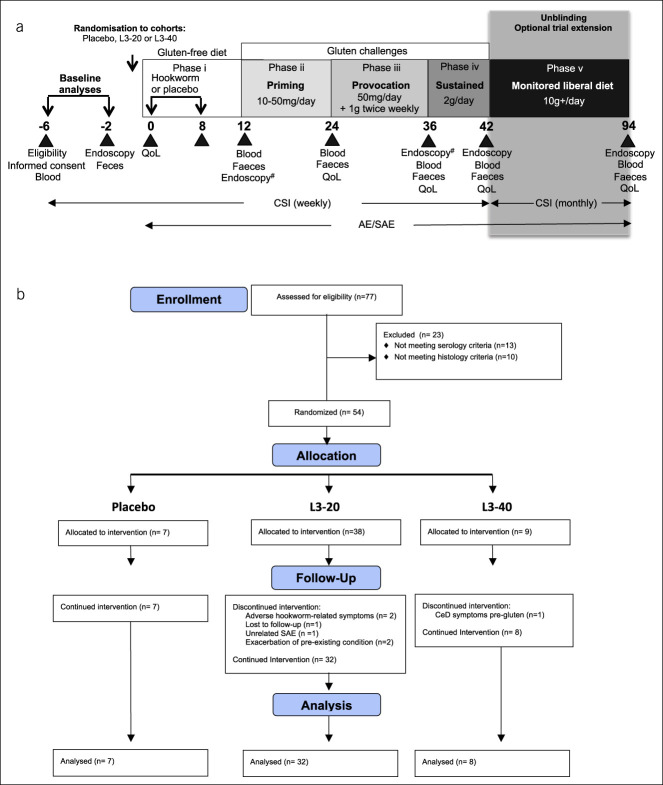
Figure 1.
Study time line, interventions, and CONSORT flow chart. (a) After screening and baseline analyses, participants with CeD were allocated to receive either placebo (chilli pepper solution) or hookworm larvae (L3-20 or L3-40) delivered over 2 occasions, at week 0 and week 8 (phase i). L3 denotes Necator americanus stage 3. At week 12, subjects received a 12-week gluten priming challenge (phase ii: 10–50 mg/d for 12 weeks), followed by a gluten provocation challenge (phase iii: 50 mg/d +1 g twice weekly for 12 weeks) and sustained gluten challenge (phase iv: 2 g/d for 6 weeks). At week 42, following evaluation of patient symptoms, the study became unblinded and participants in active L3-treated cohorts were given the option of undertaking a monitored “liberal diet” of at least 10 g of gluten/day, with freedom of food choice for 12 months (phase v). Participants underwent regular clinic visits as denoted by the black triangles, where various biological samples and survey assessments were taken for analysis of safety and CeD pathology. #Denotes endoscopy performed at week 12 instead of week 36 in the L3-40 cohort. (b) CONSORT chart showing flow of patients through the clinical trial. AE, adverse event; CeD, celiac disease; CSI, CeD symptom index survey; QoL, CeD quality of life survey; SAE, serious AE.