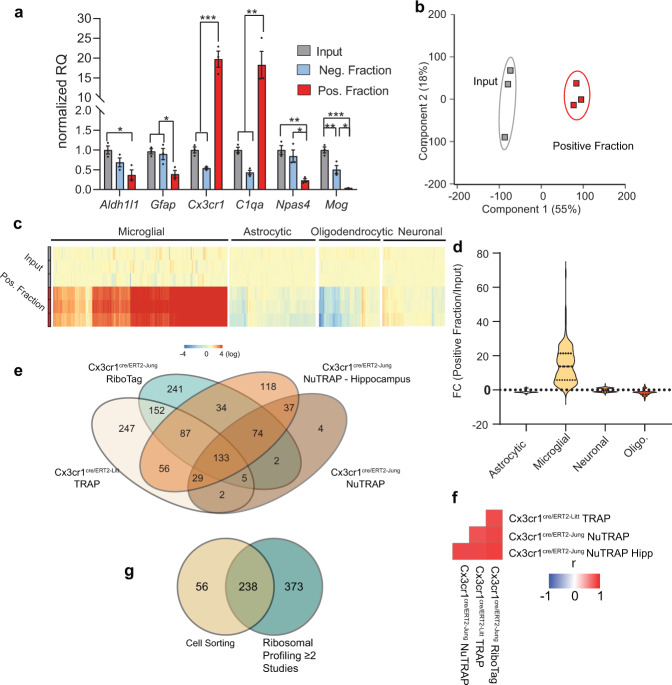
Fig. 7. The enrichment of microglial transcriptome in the Cx3cr1-NuTRAP brain is scalable to small brain regions such as hippocampus.
a TRAP-isolated RNA from input and positive fractions of dissected hippocampus were examined by qPCR for enrichment/depletion of selected cell-specific genes for astrocytes, microglia, neurons, and oligodendrocytes. Bar graphs represent average relative gene expression ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001, respectively, by one-way ANOVA with Tukey’s multiple comparison test of fractions (n = 3/group). b Principal component analysis of transcriptome profiles showed separation of positive fraction from input. c RNAseq heatmap graph of cell-type marker genes from prior cell-sorting studies shows enrichment of microglial marker genes and depletion of other cell-type markers, as compared to input. d Marker gene lists for different cell types were generated from cell-sorting studies as described in the text. Enrichment or depletion of genes from each of the lists is presented as the fold change (Positive fraction/Input). Microglial marker genes were enriched in the positive fraction while genes from other cell types were generally depleted in the positive fraction relative to input. e Microglia marker genes with FC > 5 (Positive fraction/Input) from the Cx3cr1-cre/ERT2+ model with RiboTag5, Cx3cr1-cre/ERT2+ model with TRAP3, Cx3cr1-NuTRAP hemisected brain, and Cx3cr1-NuTRAP hippocampus identifies 133 ribosomal-tagging common microglial marker genes. f Pearson correlation of the fold change (Positive fraction/Input) for all expressed genes observed in all studies have similar levels of transcriptome enrichment and depletion. g Comparison of 611 microglial markers (enriched in at least two ribosome profiling studies in e) with previously identified microglial markers from cell-sorting studies18 identifies 238 isolation method-independent microglia marker genes.