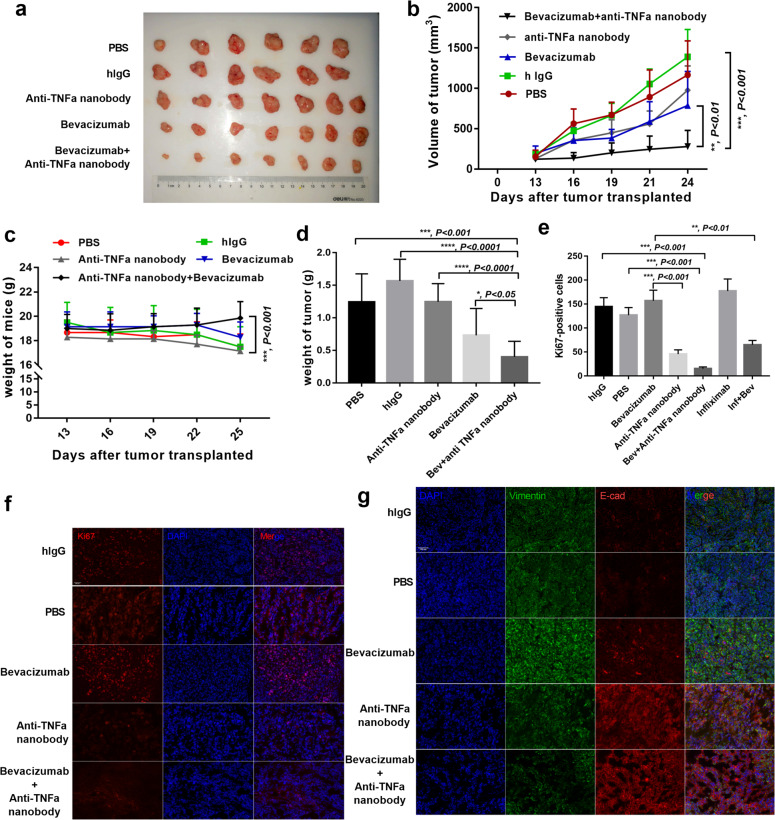
Fig. 7. Neutralizing TNFα strengthens the therapeutic effects of bevacizumab in vivo.
a Photos of MDA-MB-231-formed tumors under bevacizumab, anti-TNFα nanobody, alone or combination with both treatments. human IgG and PBS treated as control. b Tumor growth volume over time following subcutaneous transplant of tumor tissues into Balb/c nude mice. n = 7 for each condition. **P < 0.01; ***P < 0.001 by two-way ANOVA test. Error bars represent s.d. c Weight of mice over time following subcutaneous transplant of tumor tissues into Balb/c nude mice. **P < 0.01; ***P < 0.001 by two-way ANOVA test. Error bars represent s.d. d Tumor weight from humanely killed mice of each condition. *P < 0.05; ***P < 0.001; ****P < 0.0001 by t test. Error bars represent s.d. e–f Quantitative evaluation and representative images of double staining for Ki67 and nucleus. Scale bar, 200 μm. ***P < 0.001; ****P < 0.0001 by t test. Error bars represent s.d. g Representative images of tumor sections from mice treated with PBS, human IgG, bevacizumab, anti-TNFα nanobody, alone or combination with both treatments. Vimentin was co-stained with E-cadherin and nucleus. Scale bar, 200 μm.