Figure 1.
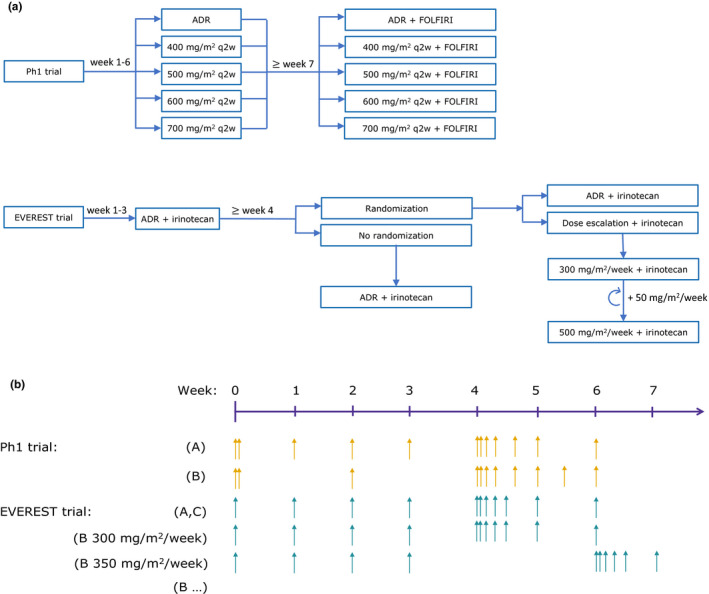
Overview of the analyzed clinical trials: a phase I (PhI) trial 7 and EVEREST trial. 8 (a) Dosing algorithm; (b) pharmacokinetic sampling schedule. The PhI trial comprised two arms. In arm A, the patients received the approved dosing regimen (ADR). In arm B, after the initial 2‐hour infusion of 400 mg/m2, the patients received 2‐hour 400 mg/m2, 2.5‐hour 500 mg/m2, 3‐hour 600 mg/m2, or 3.5‐hour 700 mg/m2 infusions q2w. In the EVEREST trial, all patients initially received cetuximab ADR in combination with irinotecan; after 3 weeks of treatment the patients eligible for randomization either continued receiving ADR (group A) or underwent dose escalation (group B), whereas the patients not eligible for randomization continued the treatment with ADR (group C). In group B, with each dose increase of 50 mg/m2 the dense sampling interval was shifted, denoted as “(B…)” for EVEREST in the figure. For all arms/groups the sampling continued until the patients dropped out of the study or until the study end. ADR, approved dosing regimen for cetuximab (2‐hour 400 mg/m2, 1‐hour 250 mg/m2 once weekly); FOLFIRI, co‐medication with irinotecan (30–90 minutes of 180 mg/m2) + 5‐fluorouracil (180 mg/m2 bolus and 2400 mg/m2 as infusion over 46 hours) + folic acid (2‐hour 400 mg/m2); q2w, every 2 weeks.
