Figure 4:
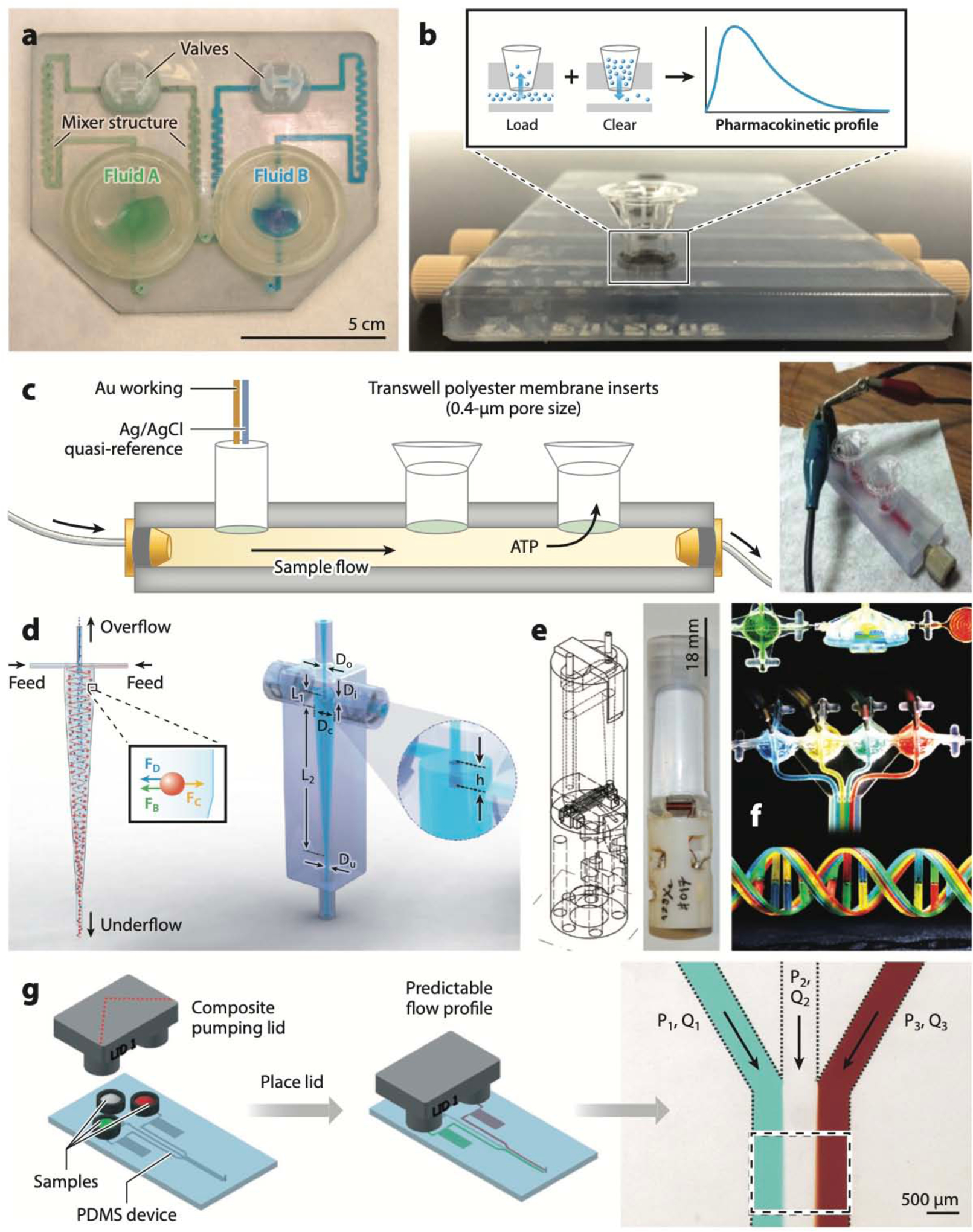
Polyjet-printed microfluidic devices. (a) Microfluidic mixer and homogenizer for DNA and protein separations (the first Polyjet-printed microfluidic device). (b) Device that interfaces with commercially available Transwell membrane inserts for measuring pharmacokinetic profiles of cells. (c) Schematic (left) and photo (right) of a device for electrochemical detection of cellular processes, where the cells are cultured inside Transwell membrane inserts. (d) A minihydrocyclone for high-throughput cell separation. (e) Schematics (left) and photograph (right) of a bubble pump for NMR applications. (f) Polyjet-printed fluidic capacitors, diodes, transistors, and a multiflow controller device. (g) A pumping lid device printed in two Polyjet resins (Tango Plus and Veroclear). Abbreviations: ATP, adenosine triphosphate; FB, buoyant force; FC, centrifugal force; FD, drag force; Dc, cylindrical diameter; Di, inlet diameter; Do, overflow diameter; Du, underflow diameter; L1, cylindrical length; L2, conical length; h, height of vortex finder; P1, pressure at inlet 1; P2, pressure at inlet 2; P3, pressure at inlet 3; PDMS, poly(dimethyl siloxane); Q1, volumetric flow rate for channel 1; Q2, volumetric flow rate for channel 2; Q3, volumetric flow rate for channel 3. Panel a adapted from Reference 129 courtesy of A. Bonyár. Panel b adapted from Reference 131 with permission from the American Chemical Society. Panel c adapted from Reference 132 with permission from the Royal Society of Chemistry (RSC). Panel d adapted from Reference 134 with permission from the RSC. Panel e adapted from Reference 136 with permission from the RSC. Panel f adapted from Reference 137 with permission from the RSC. Panel g adapted from Reference 141 with permission from the RSC.
