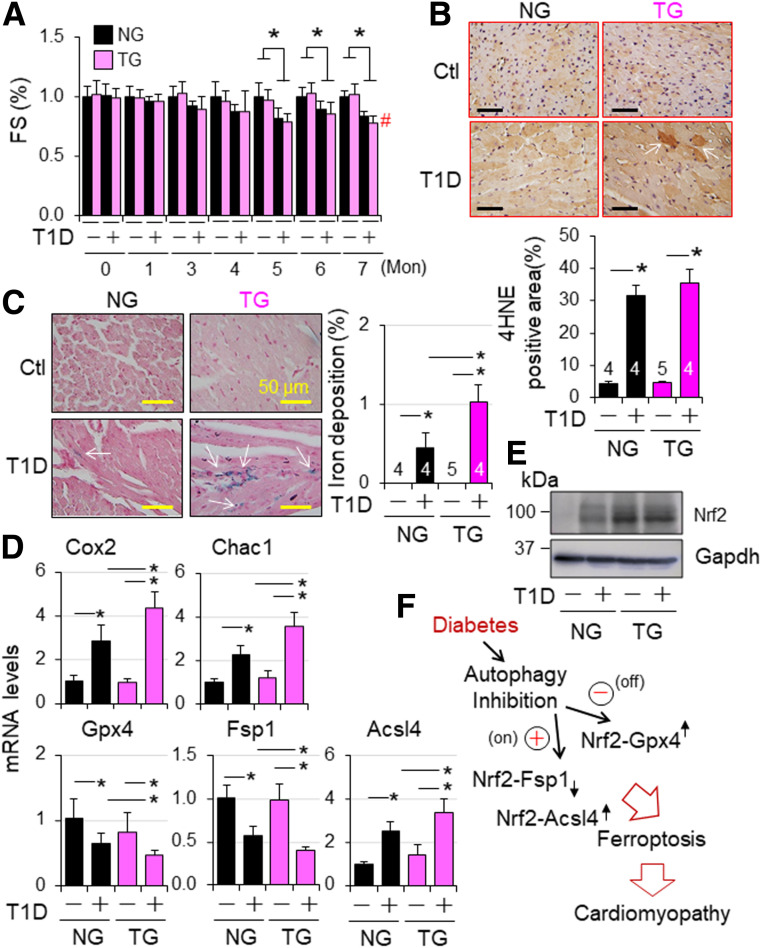
Figure 8.
Nrf2-operated myocardial anti- and proferroptotic signaling in type 1 diabetic mice. T1D in adult female NG and TG mice was induced by i.p. injection of STZ as described in research design and methods. Animal numbers of each group are shown in Supplementary Fig. 22C. A: Cardiac function. Cardiac function was monitored monthly. FS (%) in fold changes. FS (%) of vehicle-treated NG and TG groups are set as onefold. *P < 0.05 between indicate groups; #P < 0.05 vs. STZ NG group at 7 months. B: Myocardial 4HNE staining at 7 months after onset of T1D. C: Myocardia iron staining at 7 months after onset of T1D. n = 3. *P < 0.05 between indicated groups. D: The effect of CR-Nrf2 overexpression on T1D-induced ferroptotic signaling. T1D in adult male NG and TG mice was induced by i.p. injection of STZ for 6 months, and myocardial expression of ferroptotic signaling genes was assessed by qPCR analysis (n = 4). *P < 0.05 between indicated groups. E: Western blot analysis of myocardial Nrf2 expression in NG and TG mice at 6 months after onset of diabetes. The results are representatives of four separated experiments. LVs of adult male NG and TG mice at 6 months after onset of diabetes were harvested for qPCR (D) and Western blot analyses (E). F: A working hypothesis. Diabetes over time impairs myocardial autophagy, which in turn inactivates Nrf2-operated antioxidant defense, such as GPX4 expression, while intensifying an Nrf2-driven pathological gene program, such as upregulation of ACSL4 to promote lipid peroxidation for ferroptosis, thereby promoting the progression of diabetic cardiomyopathy.