FIGURE 1.
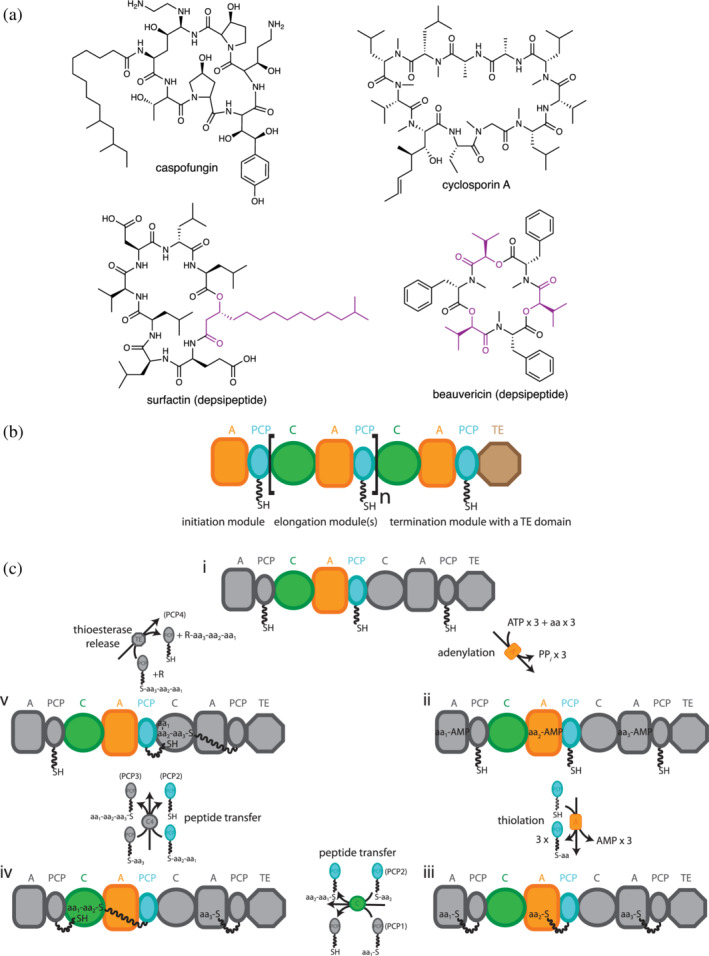
(a) Some nonribosomal peptides (top) and depsipeptides (bottom). Depsipeptides contain amide and ester bonds. Monomers from which the bridging ester oxygen originates are highlighted in purple. (b) The modular organization of nonribosomal peptide synthetases (NRPSs). Brackets highlight an elongation module. NRPS domains are: A, adenylation; C, condensation; PCP, peptidyl carrier protein; TE, thioesterase. (c) A typical NRPS synthetic cycle. The cycle starts with A domains activating monomers by adenylation, followed by their attachment to PCP domains in a phosphopantetheinyl moiety (ppant) as a thioester. PCP domains transport intermediates throughout the cycle. C domains catalyze amide (or ester, see Section 3.1) bond formation between adjacent PCP‐bound intermediates, elongating the peptide chain. Chain release occurs in termination modules, with TE domains the most common termination domains. Terminal reductase domains and terminal condensation domains also occur frequently. aa, amino acid; PPi, inorganic pyrophosphate. Adapted from Huguenin‐Dezot et al. 153
