FIGURE 6.
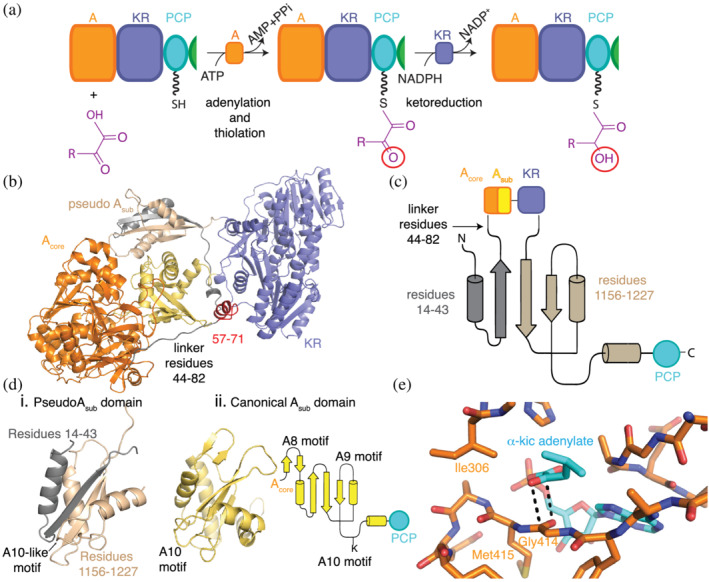
An initiation depsipeptide (A‐KR‐PCP) module. Note that elongation depsipeptide modules include the C domain (C‐A‐KR‐PCP). (a) Specialized A domains activate α‐keto acids by adenylation and attach the keto residue to the PCP domains for transport to ketoreductase (KR) domains within the same module. KR domains reduce the α‐keto group, generating α‐hydroxy acyl thioesters, which can be used in downstream reactions. Cartoon (b) and topology (c) representations show that the A domain has integral Acore (orange) and Asub (yellow) domains in the monomer structural model of the A‐KR‐PCP module of StrA from Bacillus stratosphericus LAMA 585 (based on dimeric structure PDB ID: 6ULW 34 ). A small linker connects the Asub and KR (purple) domains, followed by a small domain (wheat and gray) with a tertiary structure (d) that resembles a canonical Asub domain. (e) α‐ketoacids bind to A domains through an antiparallel carbonyl‐carbonyl interaction (PDB ID: 6ULX, 6ULY). Figure adapted from Alonzo et al. 34
