FIGURE 9.
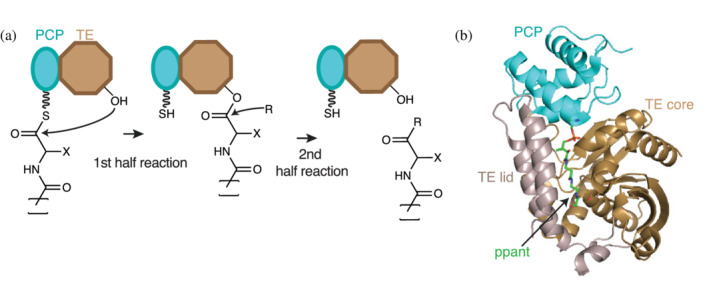
The thioesterase (TE) domain. (a) TE domains (brown) use an active site Ser (or, in rare instances, a Cys) residue to attack the elongated acyl thioester chain in the first half reaction. The resulting ester (thioester in case of Cys) is released by nucleophilic attack in the second half reaction. The nature of the incoming nucleophile determines the chemistry of the final product. (b) Cartoon representation of the holo‐PCP‐TE structure of entF (PDB ID: 3tej). 95 The TE domain has an α/β hydrolase fold formed by core (brown) and lid (grey) regions. TE lids are variable from protein to protein, and can be mobile
