FIGURE 15.
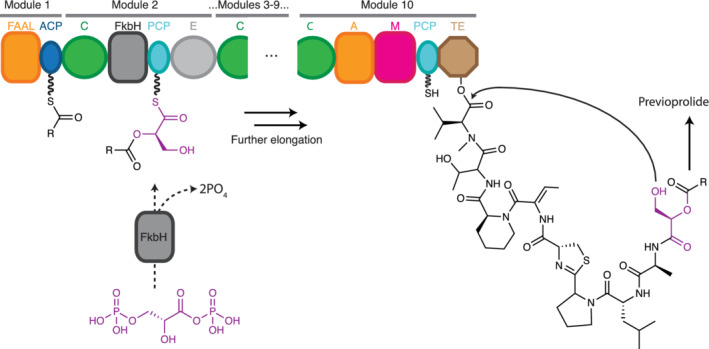
Proposed vioprolide biosynthesis. A decamodular NRPS generates previoprolide. It is suggested that the FkbH domain in Module 2 catalyzes stepwise covalent binding of D‐1,3‐biphosphoglycerate, dephosphorylation and transfer of glycerate to PCP2, generating D‐glyceryl‐S‐PCP2. The α‐hydroxy group serves as a nucleophile in the condensation reaction in the C2, and the β‐hydroxy group in the glyceryl side chain serves as the nucleophile in cyclic termination. This releases a previoprolide intermediate that undergoes further modification to generate mature vioprolide
