FIGURE 17.
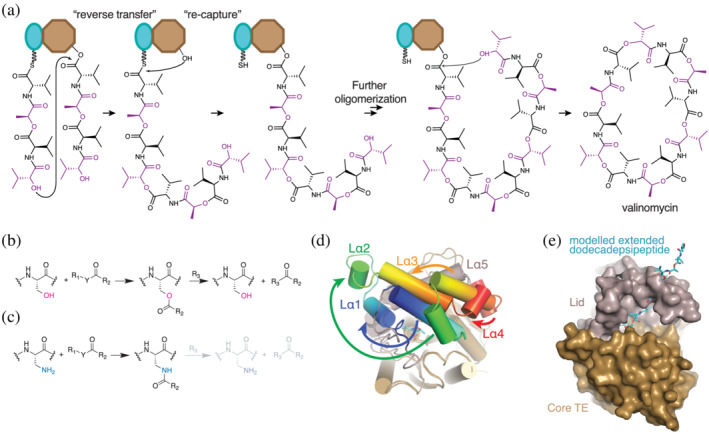
Valinomycin oligomerization and cyclization. (a) Oligomerization of tetradepsipeptidyl units proceeds through a reverse transfer pathway, where the distal α‐hydroxyl in tetradepsipeptidyl‐S‐PCP4 attacks the ester carbon in tetradepsipeptidyl‐O‐TE4, generating (tetradepsipeptidyl)2‐S‐PCP4 after one oligomerization. This is transferred to TE4 and the next tetradepsipeptidyl‐S‐PCP4 is used to make (tetradepsipeptidyl)3‐S‐PCP4 in an analogous way. Cyclization of the (tetradepsipeptidyl)3‐S‐PCP4 via its α‐hydroxyl releases mature valinomycin. (b) Vlm 2 TE forms ester intermediates during its reaction cycle. (c) Replacing the active site serine in Vlm2 TE by DAP generates a protein that can form amide bonds instead of ester bonds, making the intermediates more stable and amenable for structural and biophysical analysis. (d) The active site lid of Vlm2 TE undergoes massive conformational arrangements to favor the cyclization conformation of the dodecadepsipeptide in the active site. (e) A model of a fully elongated dodecadepsipeptide (cyan) shows that such conformation would clash with the active site lid (grey) observed in the X‐ray structure of the dodecadepsipeptidyl‐NH‐TE complex, thus supporting the hypothesis that lid remodeling favors the cyclization reaction. Figure based on Huguenin‐Dezot et al. 153
