FIGURE 2.
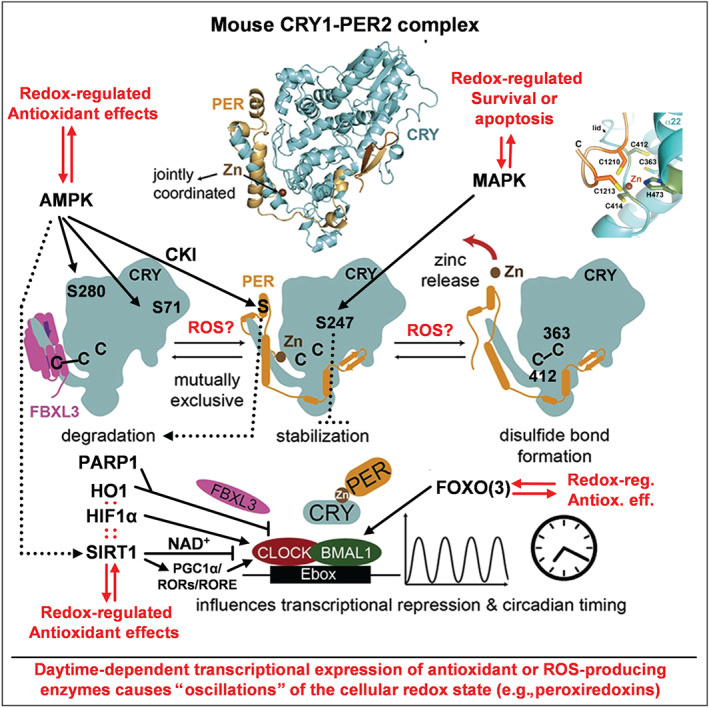
Proposed mechanisms of redox regulation of the circadian clock. A number of redox‐sensitive cysteine thiol groups (C363 and C412) and a zinc‐sulfur centre (C1210 and C1213 of PER2, C414 and H473 of CRY1, top right) were identified in mammalian CRY that act as redox switches via disulphide bond formation controlling PER binding and thereby the activity of the CLOCK/BMAL1 complex. The scheme also contains other redox‐sensitive pathways in the regulation of circadian rhythm such as redox‐sensitive kinases AMPK or MAPK. AMPK phosphorylates S71 and S280 to affect the affinity of CRY1 for the E3 ligase FBXL3 and thereby CRY stability. The MAPK phosphorylates S247 to affect CRY‐dependent transcriptional repression of BMAL1/CLOCK. Furthermore, stress response proteins such as PARP‐1, HO‐1, HIF‐1α, PGC‐1α, FOXO3, and the histone deacetylase SIRT‐1 affect the circadian clock by modifying the transcriptional activity of BMAL1/CLOCK (see main text and extended information in 2.2 in the Supporting Information for details). Vice versa, the expression of several antioxidant and ROS‐producing enzymes is controlled by the circadian clock and thereby contributes to cellular redox homeostasis. Modified from graphical abstract in Schmalen et al. (2014) with permission (Copyright © 2014 Elsevier Inc. All rights reserved)
