Abstract
BACKGROUND & AIMS:
Nonalcoholic fatty liver disease (NAFLD) is the most common pediatric chronic liver disease. Little is known about outcomes in recognized youth.
METHODS:
We compared paired liver biopsies from 122 of 139 children with NAFLD (74% male; 64% white; 71% Hispanic; mean age, 13 ± 3 years; age range, 8–17 years) who received placebo and standard of care lifestyle advice in 2 double-blind, randomized clinical trials within the nonalcoholic steatohepatitis (NASH) clinical research network from 2005 through 2015. We analyzed histologic changes with respect to baseline and longitudinal change in clinical variables using regression analysis.
RESULTS:
At enrollment, 31% of the children had definite NASH, 34% had borderline zone 1 NASH, 13% had borderline zone 3 NASH, and 21% had fatty liver but not NASH. Over a mean period of 1.6 ± 0.4 years, borderline or definite NASH resolved in 29% of the children, whereas 18% of the children with fatty liver or borderline NASH developed definite NASH. Fibrosis improved in 34% of the children but worsened in 23%. Any progression to definite NASH or in fibrosis occurred in 36% of the children, and both occurred in 11% of the children. Any improvement in NASH or fibrosis occurred in 52%, and both occurred in 20% of children. Type 2 diabetes developed in 5% of the cohort. Any progression to NASH and/or fibrosis was associated with adolescent age, higher waist circumference, levels of alanine or aspartate aminotransferase, total and low-density lipoprotein-cholesterol at baseline, increasing level of alanine aminotransferase, and hemoglobin A1C (P < .05). Progression to NASH and/or fibrosis were also associated with increasing level of gamma-glutamyl transferase and development of type 2 diabetes (P < .01). Increasing level of gamma-glutamyl transferase also associated with reduced odds of any improvement (P = .003).
CONCLUSIONS:
One-third of children with NAFLD enrolled in placebo groups of clinical trials had histologic features of progression within 2 years, in association with increasing obesity and serum levels of aminotransferases and loss of glucose homeostasis.
Keywords: ALT, Cirrhosis, Histology, Natural History
Nonalcoholic fatty liver disease (NAFLD) affects an estimated 10% of children in the United States, yet very little is known about the natural history in youth.1 Given that children have no or little exposure to alcohol yet develop NAFLD early in life, with similar histological severity to adults, it is imperative to understand the shortand long-term health outcomes both in childhood and entrance into adulthood. Complications of NAFLD in adults include cirrhosis, end-stage liver disease, and increased cardiovascular morbidity and mortality2; however, unique genetic and environmental susceptibilities associated with earlier childhood onset, potential for longer duration of disease, and a distinct periportal histological pattern in a substantial subset of children, impair extrapolation of adult outcomes to children.3,4 In children, pubertal stage also associates with differing histological patterns at diagnosis but whether outcomes differ by pubertal or gender remain unexamined.5 Approximately 15% of children in the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored NASH Clinical Research Network have advanced fibrosis at time of entry into the registry.6 Children with NAFLD exhibit increased cardiometabolic complications, including type 2 diabetes, at time of diagnosis6–8; however, the rate of regression or progression in fibrosis and other histological features are uncharacterized.
Existing literature is limited regarding the natural history of pediatric NAFLD. Feldstein et al.9 retrospectively reported on 65 children with biopsy-confirmed NAFLD over a varying number of years, with 5 undergoing repeat biopsy. Four of the 5 had worsening fibrosis, but referral and selection bias regarding re-biopsy limit generalizability. A few pediatric reports have documented rapid progression to cirrhosis, and even liver transplantation or death; however, these studies are likewise hampered by small sample size, retrospective single-center design, and ascertainment bias.10–12 No prospective pediatric reports examining histological outcomes exist.
Research in children requires that more than minimal risk be offset by likelihood of personal benefit. Thus, a natural history study in children with NAFLD with specific interval biopsy reassessment is ethically feasible only within a double-blind randomized clinical trial including a placebo group. Even this group must be provided standard of care lifestyle advice targeted at achieving a healthier weight status, the same recommendations that are provided in routine pediatric care to any child with overweight or obesity, regardless of NAFLD diagnosis.8 Two recent multicenter randomized pediatric clinical trials conducted by the NASH Clinical Research Network (NASH CRN) included a combined total of 139 children with biopsy-confirmed NAFLD who received lifestyle counseling and placebo but no other interventions.13,14 Liver biopsies were performed before enrollment as standard of care and at end-of-treatment per protocol.
Our primary aim was to determine how changes in liver histology associate with age at baseline, sex, ethnicity; and changes in body mass index (BMI), dyslipidemia, and measures of glucose homeostasis over time in children assigned to placebo receiving only standard-of-care lifestyle counseling. Second, we aimed to identify the incidence of type 2 diabetes and its relationship to histological outcomes, given the strong baseline association of prediabetes and diabetes with severity of NAFLD in children.6
Methods
Study Design and Population
Between 2005 and 2015, the NIDDK-sponsored NASH CRN conducted 2 multicenter randomized, double-blind, placebo-controlled clinical trials evaluating (1) vitamin E or metformin for the Treatment of NASH in Children (TONIC; NCT00063635), and (2) Cysteamine Bitartrate Delayed Release for the Treatment of NAFLD in Children (CyNCh; NCT01529268).13,14 These trials enrolled 8- to 17-year-old children with biopsy-confirmed NAFLD at 10 clinical centers (Appendix 1 lists participating centers and Appendix 2 inclusion/exclusion criteria and time periods for both trials). Institutional review boards at each clinical center and the data coordinating center approved both studies. Independent data and safety monitoring boards appointed by the NIDDK regularly monitored data quality and patient safety. Parents or guardians provided written consent and all children provided written assent.
After randomization, all participants received standardized nutrition and exercise counseling (Appendix 3) consistent with standard of care American Academy of Pediatrics Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity at each visit, at approximately 12-week intervals.15
Inclusion criteria for this study were enrollment in the TONIC or CyNCh placebo arms with paired liver biopsies at enrollment and end-of-treatment at 96 or 52 weeks, respectively (CONSORT diagram shown in Supplementary Figure 1). Children were assigned placebo capsules matching the treatment medications. Children receiving active treatment were excluded. Standard laboratory evaluations and expert liver histology reviews excluded other liver diseases. Participants without end-of-treatment liver biopsies were excluded. The writing group prepared the manuscript, which was approved by the NASH CRN Publications Committee and the Steering Committee.
Clinical and Demographic Measures
Age, sex, race, and ethnicity were recorded at enrollment. Baseline and follow-up visits assessed medical history; anthropometric measurements of height, weight, BMI, BMI z-score, waist and hip measurements; serum hepatic panel (U/L) including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), uric acid (mg/dL), hemoglobin A1C (HbA1c, %); fasting lipid profile (mg/dL) including serum triglycerides, total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein cholesterol; fasting glucose and insulin (μU/mL).
Participants and caregivers completed the Pediatric Quality of Life Inventory, v 4.0, with higher scores indicating better health-related quality of life (http://pedsql.org/index.html). The end-of-treatment visit (including biopsy) was performed at 96 weeks in TONIC and 52 weeks in CyNCh. A final visit off treatment occurred at 120 weeks in TONIC and 76 weeks in CyNCh. No participants in this study were enrolled in both trials.
Baseline comorbid conditions (dyslipidemia, hypertension, and type 2 diabetes) were assessed by trained study staff. Incident type 2 diabetes was defined as a new diagnosis of type 2 diabetes obtained and verified during interim medical visits by study staff and/or HbA1c ≥6.5%.
Liver Histology
All baseline and end-of-treatment liver biopsies were reviewed centrally and scored by the NASH CRN Pathology Committee.13,14 Composite histologic activity was assessed using the validated NAFLD Activity Score (NAS) on a scale from 0 to 8, with higher scores indicating more severe disease.16 The component measures of the NAS include steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2) grades. Fibrosis was staged on a scale of 0 to 4, and portal inflammation on a scale of 0 to 2. Biopsies were categorized as follows: “NAFL-not NASH”; “borderline zone 1 pattern”, “borderline zone 3 pattern”; or “definite steatohepatitis” based on the pattern of injury and presence and degree of individual lesions.17 Although no single histologic feature is considered diagnostic of NASH, typical minimum criteria for definite NASH include >5% steatosis, lobular inflammation, and evidence of hepatocellular ballooning. Borderline zone 3 pattern has some features of definite NASH, but does not meet full criteria, whereas borderline zone 1 pattern has portal predominant lesions and is more common in children.4 Biopsies with abnormal steatosis (>5%), but without inflammation or ballooning consistent with definite or borderline NASH were designated “NAFL-not NASH.”
Definition of Histological Outcomes
Resolution of NASH was defined as change from baseline borderline or definite NASH to NAFL-not NASH or complete resolution of NAFLD at end-of-treatment. Histological progression to definite NASH was defined as change from baseline “NAFL-not NASH” or borderline NASH to definite NASH. Further, the change from a predominantly pediatric pattern of NASH to an adult pattern was defined as change from borderline zone 1 NASH to borderline zone 3 or to definite NASH at end-of-treatment.
Regression of fibrosis was defined as ≥1 point decrease in fibrosis stage from baseline. Progression of fibrosis was defined as ≥1 point increase in fibrosis stage from baseline.
Composite histological improvement was defined as resolution of NASH and regression of fibrosis at end-of-treatment. Any improvement in disease was defined as resolution of NASH and/or regression of fibrosis at end-of-treatment (either or both). Composite progression in disease was defined as ≥1 stage increase in fibrosis and progression to definite NASH at end-of-treatment. Any progression in disease was defined as ≥1 stage increase in fibrosis and/or progression to definite NASH at end-of-treatment (either or both).
Changes in steatosis, lobular and portal inflammation, and ballooning were defined as improvement if ≥1 point decrease in score at end-of-treatment from baseline, and progression if ≥1 point increase in score at end-of-treatment from baseline. The composite change in NAS and the changes in individual histological feature scores and fibrosis staging were analyzed as the net change in value at end-of-treatment compared with baseline.
Statistical Analysis
Due to the potential influence of sex hormones, baseline and longitudinal changes in characteristics were compared between boys and girls. Similarly, because differences in NAFLD histology have been associated with pubertal transition from child to adolescent ages, characteristics were also compared between pre-adolescent (8–12 years) vs adolescent age groups (13–17 years).5
Baseline characteristics were compared for differences in distributions within these subgroups using either a Fisher’s exact test (when categorical) or a t test (if continuous). To assess the longitudinal changes in binary characteristics by each subgroup (sex or age group), logistic regression models of the change at end of follow-up for each binary characteristic in relationship to the subgroup were used. For continuous characteristics, P values and adjusted mean changes from baseline by each subgroup were computed using analysis of covariance, regressing change from baseline to end of follow-up on each specified subgroup and the baseline value of the characteristic.
Twenty-seven demographic and clinical characteristics (each at baseline and changes at end of follow-up) were analyzed for associations with 6 histological outcomes using logistic regression models of the histological outcome in relation to the characteristic. Patatin-like phospholipase domain-containing protein 3 (PNPLA3) genotype data were available in 80% of the cohort (n = 97) and included in the analysis. Distribution of a categorized BMI z-score change was examined by increments of 0.25 unit increase or decrease. Incident rate of diabetes was calculated as the number of participants with incident diabetes over period of follow-up through posttreatment visit divided by the person-years of follow-up, multiplied by 1000. An exact logistic regression was used to analyze incident diabetes with 4 of the outcomes due to small numbers and completely determined outcomes. Patterns of progression (1) to definite NASH, and (2) of fibrosis were shown graphically for ALT, AST, GGT, LDL, HbA1c, and BMI z-score. Generalized estimating equations (GEE) were used to account for repeated visits per child for multiple linear regression models of the change in laboratory test or BMI z-score in relation to the histological outcome indicator, adjusted for the baseline value of the laboratory test or BMI z-score, the visit code, and the interaction of visit by histological outcome, respectively; the test for a trend over time was determined by a Wald test of the outcome effect and the interaction term the histology indicator and time.
All statistical analyses were performed using SAS Statistical Software version 9.4 (SAS Institute, Inc., Cary, NC) and Stata (release MPv16 ; StataCorp LP, College Station, TX).
Results
Baseline Characteristics of Study Participants
Paired liver biopsies were obtained in 47 of 58 subjects in the placebo arm of TONIC and 75 of 81 subjects in the placebo arm of CyNCh, or 88% (122/139) of all participants in the placebo arms. Baseline characteristics of the combined 122 participants are presented in Table 1. The cohort had a mean age of 13 ± 3 years (range 8–17 years), and was 74% male, 64% white race, and 71% Hispanic ethnicity, consistent with known trends of higher prevalence of NAFLD in youth in these demographic groups. Among those of Hispanic ethnicity, 56% were of self-reported white race and 44% of other racial backgrounds. At time of enrollment, 7% of the cohort had a diagnosis of type 2 diabetes, 11% had hyperlipidemia, and 7% had high blood pressure.
Table 1.
Baseline demographic, clinical, and histological characteristics in children with NAFLD by sex and age
| Totala |
Sex |
Age group |
|||||
|---|---|---|---|---|---|---|---|
| Characteristics | Children (N = 122) | Male (n = 90) | Female (n = 32) | Pb | 13–17 (n = 62) | 8–12 (n = 60) | Pb |
| Demographic: | |||||||
| Sex: male, n (%) | 90 (74) | n/a | n/a | 47 (76) | 43 (72) | .68 | |
| Age group: 8–12 y, n (%): | 62 (51) | 47 (52) | 15 (47) | .68 | n/a | n/a | |
| Mean age (SD), y | 13.3 (2.6) | 13.5 (2.4) | 12.9 (2.9) | .28 | 15.5 (1.5) | 11.1 (1.2) | <.001 |
| Hispanic, n (%) | 87 (71) | 63 (70) | 24 (75) | .66 | 35 (56) | 52 (87) | <.001 |
| Race: White, n (%) | 78 (64) | 59 (66) | 19 (59) | .53 | 45 (73) | 33 (55) | .06 |
| Race/Ethnicityc: n (%) | .70 | .002 | |||||
| Non-Hispanic White | 29 (24) | 31 (34) | 13 (41) | 23 (37) | 6 (10) | ||
| Hispanic White | 49 (40) | 36 (40) | 13 (41) | 22 (35) | 27 (45) | ||
| Other: any other race & ethnicity | 44 (36) | 23 (26) | 6 (19) | 17 (27) | 27 (45) | ||
| PNPLA3 genotypec: n (%) | .56 | .06 | |||||
| CC | 50 (52) | 34 (48) | 16 (62) | 26 (55) | 24 (48) | ||
| CG | 38 (39) | 30 (42) | 8 (31) | 20 (43) | 18 (36) | ||
| GG | 9 (9) | 7 (10) | 2 (8) | 1 (2) | 8 (16) | ||
| Anthropometric/physical: | |||||||
| Body Mass Index (BMI, kg/m2) | 32.3 (5.5) | 32.2 (5.1) | 32.7 (6.6) | .71 | 29.9 (5.1) | 34.7 (4.9) | <.001 |
| Body Mass Index (BMI) z-score, n (%): | |||||||
| 2.5+ (severely obese) | 31 (25) | 25 (28) | 6 (19) | .35 | 16 (26) | 15 (25) | 1.00 |
| Mean (SD) | 2.2 (0.4) | 2.3 (0.4) | 2.2 (0.4) | .37 | 2.3 (0.4) | 2.2 (0.4) | .43 |
| BMI (for sex-age) %ile: mean (SD) | 98.1th (2.8) | 98.2th (2.6) | 97.8th (3.3) | .47 | 98.0th (2.8) | 98.2th (2.8) | .65 |
| Blood Pressure (BP): | |||||||
| Systolic BP (mm Hg) | 122.1 (13.7) | 123.0 (14.2) | 119.3 (11.9) | .19 | 124.9 (13.3) | 119.2 (13.5) | .02 |
| Diastolic BP (mm Hg) | 67.0 (9.4) | 68.1 (9.2) | 64.1 (9.3) | .04 | 68.9 (9.8) | 65.1 (8.6) | .02 |
| Blood Pressure %tiles: mean (SD) | |||||||
| Systolic BP (adj sex-age-ht) | 76.7th (25.0) | 75.6th (26.1) | 79.7th (21.5) | .43 | 77.9th (24.7) | 75.4th (25.4) | .59 |
| Diastolic BP (adj sex-age-ht) | 57.3th (25.2) | 60.0th (24.5) | 49.8th (26.2) | .049 | 56.1th (23.4) | 58.3th (27.0) | .63 |
| Comorbidity, n (%): | |||||||
| Any diabetesd | 8 (7) | 3 (3) | 5 (16) | .03 | 7 (11) | 1 (2) | .06 |
| Any hyperlipidemia diagnosis | 13 (11) | 7 (8) | 6 (19) | .10 | 7 (11) | 6 (10) | 1.00 |
| Any high blood pressure diagnosis: | 9 (7) | 6 (7) | 3 (9) | .70 | 7 (11) | 2 (3) | .16 |
| Quality of Life (QOL): | |||||||
| Self-reported pediatric QOL | |||||||
| Physical health | 79 (20) | 79 (20) | 77 (18) | .58 | 80 (21) | 78 (19) | .52 |
| Psychosocial health | 74 (17) | 74 (18) | 71 (17) | .41 | 72 (18) | 75 (17) | .47 |
| Parent/Guardian-reported pediatric QOL | |||||||
| Physical health | 67 (24) | 68 (25) | 65 (21) | .52 | 68 (24) | 66 (19) | .79 |
| Psychosocial health | 65 (20) | 65 (20) | 66 (10) | .92 | 64 (20) | 61 (19) | .64 |
| Laboratory: | |||||||
| ALT (U/L) | 112.4 (71.3) | 111.1 (69.4) | 116.0 (77.4) | .74 | 104.5 (66.1) | 120.8 (76.0) | .21 |
| AST (U/L) | 65.1 (38.0) | 62.6 (33.8) | 72.1 (47.8) | .23 | 60.4 (36.0) | 69.9 (39.7) | .17 |
| GGT (U/L) | 47.2 (30.5) | 47.6 (28.9) | 46.1 (35.2) | .81 | 43.6 (25.7) | 51.0 (34.7) | .18 |
| Cholesterol, total (mg/dL) | 168.7 (35.9) | 168.0 (34.6) | 170.6 (39.9) | .73 | 170.0 (38.8) | 167.4 (32.9) | .69 |
| Cholesterol, HDL (mg/dL) | 40.7 (9.5) | 41.3 (9.8) | 38.8 (8.4) | .20 | 38.9 (6.8) | 42.5 (11.4) | .04 |
| Cholesterol, LDL (mg/dL) | 98.7 (30.6) | 99.0 (29.5) | 97.7 (33.8) | .84 | 99.5 (31.7) | 97.8 (29.5) | .76 |
| Triglycerides (mg/dL) | 150.5 (77.7) | 143.4 (78.2) | 170.4 (73.9) | .10 | 158.9 (79.0) | 141.8 (76.0) | .23 |
| Uric acid (mg/dL) | 5.84 (1.45) | 6.00 (1.53) | 5.39 (1.12) | .04 | 6.42 (1.52) | 5.23 (1.09) | <.0001 |
| Glucose (mg/dL) | 88.7 (12.2) | 88.4 (10.1) | 89.8 (16.9) | .56 | 89.6 (14.7) | 87.8 (9.1) | .41 |
| HOMA-IR (mg/dL X μU/mL/405) | 9.6 (13.1) | 9.0 (14.0) | 11.0 (10.3) | .47 | 11.9 (17.5) | 7.1 (4.5) | .047 |
| HbA1c (%) | 5.4 (0.4) | 5.4 (0.4) | 5.5 (0.4) | .13 | 5.4 (0.5) | 5.4 (0.3) | .71 |
| MRI (CyNCH onlyd) | |||||||
| PDFF (%) | 21.5 (9.6) | 21.5 (10.1) | 21.7 (8.7) | .95 | 19.6 (10) | 23.7 (8.9) | .11 |
| Histological features: | |||||||
| Fibrosis stage, n (%): | |||||||
| None (0) | 34 (28) | 24 (27) | 10 (31) | .64 | 20 (32) | 14 (23) | .02 |
| Mild (1a/b/c) | 53 (43) | 42 (47) | 11 (34) | 27 (44) | 26 (43) | ||
| Moderate (2) | 18 (15) | 12 (13) | 6 (19) | 12 (19) | 6 (10) | ||
| Bridging/cirrhosis (3,4)c | 17 (14) | 12 (13) | 5 (16) | 3 (5) | 14 (23) | ||
| Mean (SD) | 1.1 (1.0) | 1.1 (1.0) | 1.2 (1.1) | .79 | 1.0 (0.8) | 1.3 (1.1) | .04 |
| NASH diagnosis, n (%): | .55 | <.001 | |||||
| None | 26 (21) | 18 (20) | 8 (25) | 16 (26) | 10 (17) | ||
| Borderline Zone 3 | 16 (13) | 10 (11) | 6 (19) | 9 (15) | 7 (12) | ||
| Borderline Zone 1 | 42 (34) | 33 (37) | 9 (28) | 8 (13) | 34 (57) | ||
| Definite NASH | 38 (31) | 29 (32) | 9 (28) | 29 (47) | 9 (15) | ||
| NAFLD Activity Score (NAS) | 4.6 (1.4) | 4.6 (1.4) | 4.8 (1.4) | .46 | 4.7 (1.5) | 4.6 (1.3) | .57 |
| Ballooning score (0–2), n (%): | |||||||
| Few/many (1,2) | 57 (47) | 42 (47) | 15 (47) | 1.00 | 38 (61) | 19 (32) | .001 |
| Mean (SD) | 0.6 (0.8) | 0.6 (0.7) | 0.7 (0.8) | .68 | 0.9 (0.8) | 0.4 (0.7) | .001 |
| Steatosis grade (1–3), n (%): | |||||||
| Mild | 21 (17) | 15 (17) | 6 (19) | .37 | 14 (23) | 7 (12) | .02 |
| Moderate | 39 (32) | 26 (29) | 13 (41) | 24 (39) | 15 (25) | ||
| Severe | 62 (51) | 49 (54) | 13 (41) | 24 (39) | 38 (63) | ||
| Mean (SD) | 2.3 (0.8) | 2.4 (0.8) | 2.2 (0.8) | .31 | 2.2 (0.8) | 2.5 (0.7) | .009 |
| Lobular inflammation score (0–3), n (%):: | |||||||
| ≥2 under 20X (2,3) | 67 (55) | 45 (50) | 22 (69) | .10 | 34 (55) | 33 (55) | 1.00 |
| Mean (SD) | 1.6 (0.7) | 1.6 (0.6) | 1.9 (0.7) | .02 | 1.7 (0.7) | 1.6 (0.6) | .61 |
| Chronic portal inflammation (0–2), n (%): | |||||||
| More than mild (2) | 113 (93) | 82 (91) | 31 (97) | .44 | 53 (85) | 60 (100) | .003 |
| Mean (SD) | 1.1 (0.5) | 1.0 (0.5) | 1.2 (0.5) | .11 | 1.0 (0.5) | 1.2 (0.4) | .01 |
Data are means ± standard deviations unless otherwise noted. Children in either the TONIC or CyNCh trial in the placebo group with paired baseline and end-of-treatment biopsies were included in the analyses. The follow-up period to the 2nd biopsy was 96 weeks for TONIC and 52 weeks for CyNCh.
The significance of difference in categorical variables between groups within each subgroup was tested with a Fisher’s Exact test; continuous variables were analysed with a t test. All P values are 2-sided.
The distribution of the Other race/ethnicity category is as follows: 1 Non-Hispanic Black, 2 Hispanic Blacks, 5 Non-Hispanic other race, including mixed, 34 Hispanic other race, including mixed, 2 Hispanic with missing race. 25/122 (20%) of the children were not genotyped. No children had cirrhosis at enrollment.
Diabetes at baseline determined by case review using the definition of either a doctor-diagnosis by self-report (7 reported) or by an HbA1c at visit ≥ 6.5% (1 patient was prescribed treatment before their f24 follow-up visit). An enrollment magnetic resonance examination was available for 55 children in CyNCh placebo group.
At baseline, 31% of the cohort had definite NASH, 13% borderline zone 3 NASH, 34% borderline zone 1 NASH, and the remaining 21% NAFL-not NASH. Fibrosis distribution was 28% with no fibrosis, 43% with mild stage 1 fibrosis, 15% with moderate stage 2 fibrosis, and 14% with bridging fibrosis. Per protocol, no participants had cirrhosis at entry. Mean NAS was 4.6 ± 1.4.
Distribution into preadolescent (49%) and adolescent (51%) age groups was similar at time of enrollment. Children ≤12 years had a higher proportion of Hispanic ethnicity (P < .001), lower mean uric acid and homeostatic model assessment–insulin resistance (HOMA-IR) values (P < .05), and higher high-density lipoprotein-cholesterol levels compared with adolescents ≥13 years. The pre-adolescent group also demonstrated a trend toward a higher proportion with the homozygous higher risk PNPLA3 GG genotype (16% vs 2%, P = .06). The preadolescent age group had a higher proportion with bridging fibrosis (23% vs 5%, P=.02) and severe steatosis (63% vs 39%, P=.02), although lower prevalence of hepatocellular ballooning (32% vs 61% (P = .001). Portal inflammation was common in both age groups, but universally prevalent in the children 12 years and younger (P = .003). Minimal differences emerged by sex: girls had lower mean diastolic blood pressure percentiles (P < .05) and slightly higher mean lobular inflammation score (P = .02) vs boys.
Baseline self-reported quality of life scores (physical and psychosocial health) for participants were higher than the proxy scores reported by parents/guardians, with no significant differences by age or sex groups.
Changes in Clinical Characteristics Over Period of Observation
Table 2 shows changes in clinical characteristics. Over a mean of 1.8 ± 0.4 years of observation per child (from baseline to final follow-up visit), mean BMI z-score remained unchanged, with approximately half (55%, n = 66) increasing in BMI z-score. Degree of BMI z-score change was modest with 86% remaining within ± 0.25 of baseline; BMI z-score declined ≥0.25 in only 10 children (8%) and increased by ≥0.25 in only 7 (6%). Mean serum aminotransferase levels (ALT, AST) declined significantly (P < .01) but mean GGT level did not. There were no other significant changes in clinical measures, including in quality-of-life measures. When examined by age group, preadolescents were more likely to have reduced ALT, total cholesterol, and diastolic blood pressure over time (all P < .05), compared with adolescents.
Table 2.
Changes from baseline at follow-up in clinical characteristics and histological outcomes in children with NAFLD by sex and age
| Changes from baseline |
Changes from baselinea |
Male vs Female |
Changes from baselinea |
Adolescents vs Pre |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Changes from baseline in characteristics | Total Children (N = 122) | Male (n = 90) | Female (n = 32) | OR or adjusted differences in mean changesb (95% CI) | Pb | Adolescent 13–17 y (n = 62) | Pre-adolescent 8–12 y (n = 60) | OR or adjusted differences in mean changesb (95% CI) | Pb |
| Clinical characteristics | |||||||||
| ALT (U/L) | −19.9 (77.5) | −21.2 (76.1) | −16.0 (82.3) | −8.3 ( −34.4 to 17.8) | .53 | −2.7 (75.3) | −37.9 (76.3) | 25.4 (2.6 to 48.1) | .03 |
| AST (U/L) | −10.9 (39.6) | −11.4 (35.4) | −9.3 (50.1) | −7.8 (−21.3 to 5.6) | .25 | −3.8 (37.9) | −18.2 (40.3) | 8.9 (−2.9 to 20.7) | .14 |
| GGT (U/L) | −2.7 (20.5) | −2.7 (19.4) | −2.5 (23.6) | −0.1 (−8.2 to 8.2) | 1.00 | 0.9 ((17.1) | −6.4 (23.2) | 6.1 (−1.1 to 13.3) | .09 |
| Cholesterol, total (mg/dL) | −5.6 (23.5) | −6.5 (24.0) | −2.9 (22.3) | −4.3 (−13.2 to 4.6) | .34 | −1.7 (22.7) | −9.6 (23.9) | 8.5 (0.8 to 16.2) | .03 |
| Cholesterol, HDL(mg/dL) | −1.4 (7.5) | −1.9 (7.9) | −0.2 (6.0) | 1.0 (−3.8 to 1.8) | .50 | −0.5 (7.0) | −2.4 (7.9) | 0.8 (−1.7 to 3.3) | .54 |
| Cholesterol, LDL (mg/dL) | −4.8 (21.4) | −5.5 (22.3) | −2.5 (18.9) | −2.5 (−10.7 to 5.7) | .55 | −1.7 (22.3) | −8.0 (20.2) | 6.9 (−0.12 to 14.0) | .05 |
| Triglycerides (mg/dL) | 9.1 (66.2) | 9.1 (63.6) | 9.1 (74.1) | −4.1 (−31.4 to 23.2) | .77 | 6.1 (60.9) | 12.2 (71.9) | −3.5 (−27.5 to 20.6) | .78 |
| Uric acid (mg/dL) | 0.31 (1.13) | 0.39 (1.20) | 0.09 (0.85) | 0.58 (0.20 to 0.97) | .003 | 0.04 (1.18) | 0.61 (0.99) | −0.10 (−0.48 to 0.28) | .60 |
| Glucose (mg/dL) | 4.5 (26.3) | 4.0 (23.0) | 5.6 (34.0) | −2.0 (−12.8 to 8.l8) | .71 | 5.1 (29.9) | 3.8 (22.2) | 1.7 (−7.8 to 11.3) | .88 |
| HOMA-IR (mg/dL X μU/mL/405) | 1.8 (17.6) | 1.6 (19.3) | 2.2 (12.3) | −2.2 (−8.2 to 3.8) | .48 | −0.3 (19.5) | 3.9 (15.3) | −0.4 (−5.9 to 5.0) | .88 |
| HbA1c (%) | 0.3 (1.0) | 0.3 (1.1) | 0.4 (0.8) | −0.1 (−0.5 to 0.3) | .67 | 0.4 (1.4) | 0.1 (0.5) | 0.3 (−0.04 to 0.7) | .08 |
| Anthropometric/physical: | |||||||||
| Body Mass Index (kg/m2) | 1.35 (2.53) | 1.14 (2.56) | 1.93 (2.30) | −0.8 (−1.8 to 0.2) | .13 | 1.38 (3.12) | 1.32 (1.75) | 0.2 (−0.8 to 1.2) | .72 |
| BMI z-score (category)c | |||||||||
| ≥ −0.25 decrease | 10 (8%) | 7 (8%) | 3 (10%) | 1.21 (0.31 to 4.71) | .17 | 6 (10%) | 4 (7%) | 0.67 (0.15 to 2.92) | .24 |
| >0 to −0.25 decrease | 43 (36%) | 29 (33%) | 14 (44%) | 0.58 (0.27 to 1.27) | .78 | 18 (30%) | 25 (42%) | 0.60 (0.15 to 2.93) | .60 |
| (Reference (≥ 0 higher)) | 67 (56%) | 52 (59%) | 15 (47%) | 1.00 | 37 (61%) | 30 (51%) | 1.00 | ||
| BMI (for sex-age) %tile: mean (SD) | −0.4 (2.6) | −0.1 (1.7) | −1.1 (4.2) | 0.8 (−0.2 to −1.8) | .10 | −0.2 (1.9) | −0.6 (3.2) | 0.4 (−0.5 to 1.2) | .40 |
| Blood Pressure (BP): | |||||||||
| Systolic BP (mm HG) | 0.5 (12.8) | 0.7 (13.1) | 0.2 (12.0) | 2.6 (−2.0 to 7.2) | .26 | 0.4 (12.7) | 0.5 (13.0) | 2.7 (−1.3 to 6.8) | .19 |
| Diastolic BP (mm HG) | −0.1 (9.2) | −0.7 (8.9) | 1.4 (10.1) | 0.3 (−2.8 to 3.3) | .87 | 0.2 (9.7) | 0.5 (8.7) | 3.2 (0.6 to 5.8) | 0.2 |
| Blood Pressure %iles: mean (SD) | |||||||||
| Systolic BP (adj sex-age-ht) | −4.3 (28.7) | −4.4 (28.3) | −4.0 (31.1) | −2.6 (−13.1 to 7.9) | .62 | −3.6 (30.4) | −5.1 (27.1) | 0.2 (−9.0 to 9.5) | .96 |
| Diastolic BP (adj sex-age-ht) | −3.4 (27.0) | −5.7 (25.5) | 2.9 (30.4) | −1.8 (−10.9 to 7.3) | .70 | −4.0 (28.7) | −2.8 (25.4) | 0.6 (−7.2 to 8.5) | .87 |
| Comorbidities-incident cases | |||||||||
| Diabetes type 2 incident casesd (n = 6) | 6 (5%) | 3 (3%) | 3 (9%) | 0.3 (0.1 to 1.7) | .19 | 5 (8%) | 1 (2%) | 5.2 (0.6 to 45.7) | .14 |
| Quality of Life (QOL) | |||||||||
| Self-reported pediatric QOL | |||||||||
| Physical health | 4.7 (17.9) | 4.7 (17.6) | 4.9 (18.9) | 1.2 (−4.5 to −0.5) | .69 | 2.5 (19.5) | 7.0 (15.9) | −3.2 (−8.1 to 1.7) | .20 |
| Psychosocial health | 5.1 ( −16.3) | 4.3 (16.9) | 7.4 (14.2) | −1.5 (−6.9 to 3.9) | .58 | 6.5 (15.2) | 3.6 (17.3) | 1.6 (−3.1 to 6.4) | .49 |
| Parent/Guardian-reported pediatric QOL | |||||||||
| Physical health | 3.4 (22..7) | 2.6 (23.7) | 7.8 (19.3) | −3.9 (−12.3 to 4.5) | .36 | 3.2 (24.4) | 4.8 (21.0) | −1.1 (−8.5 to 6.3) | .76 |
| Psychosocial health | 5.1 (22.0) | 4.3 (23.1) | 7.3 (18.7) | −3.2 (−11.0 to 4.5) | .41 | 7.1 (22.1) | 2.9 (21.9) | 3.2 (−3.6 to 10.0) | .35 |
| MRI: PDFF (%)d | −2.6 (8.4) | −2.9 (8.4) | −1.8 (8.7) | −2.2 (−7.4 to 2.9) | .39 | −1.0 (7.9) | −4.3 (8.8) | 0.9 (−4.0 to 5.9) | .70 |
| Changes from baseline in histological featurese | |||||||||
| Resolution of NASH (n = 96) | 28 (29%) | 21 (29%) | 7 (29%) | 1.0 (0.4 to 2.8) | 1.00 | 8 (17%) | 20 (40%) | 0.3 (0.1 to 0.8) | .02 |
| Progression to definite NASH (n = 84) | 15 (18%) | 11 (18%) | 4 (17%) | 1.0 (0.3 to 3.7) | .94 | 7 (21%) | 8 (16%) | 1.4 (0.5 to 4.5) | .52 |
| Combined improvement in: | |||||||||
| Fibrosis or NASH resolution (n = 99) | 51 (52%) | 38 (51%) | 13 (52%) | 1.0 (0.4 to 2.4) | .96 | 20 (42%) | 31 (61%) | 0.5 (0.2 to 1.0) | .06 |
| Fibrosis and NASH resolution (n = 96) | 19 (20%) | 16 (22%) | 3 (12%) | 2.0 (0.5 to 7.6) | .31 | 6 (13%) | 13 (26%) | 0.4 (0.1 to 1.2) | .12 |
| Combined progression in: | |||||||||
| Fibrosis or to definite NASH (n = 95) | 34 (36%) | 25 (36%) | 9 (26%) | 1.0 (0.4 to 2.6) | .98 | 21 (49%) | 13 (25%) | 2.9 (1.2 to 6.8) | .02 |
| Fibrosis and to definite NASH (n = 84) | 9 (11%) | 7 (11%) | 2 (9%) | 1.4 (0.3 to 7.1) | .71 | 4 (12%) | 5 (10%) | 1.3 (0.3 to 5.1) | .74 |
| Fibrosis | |||||||||
| Patients with improvement | 42 (34%) | 33 (37%) | 9 (28%) | 1.5 (0.1 to 3.6) | .38 | 18 (29%) | 24 (40%) | 0.6 (0.3 to 1.3) | .20 |
| Patients with worse fibrosis | 28 (23%) | 21 (23%) | 7 (22%) | 1.1 (0.4 to 2.9) | .87 | 18 (29%) | 10 (17%) | 2.0 (0.9 to 4.9) | .11 |
| Change in score | −0.1 (1.1) | −0.1 (1.1) | −0.1 (1.1) | −0.0 (−0.4 to 0.4) | .86 | 0.1 (1.0) | −0.3 (1.1) | 0.3 (−0.1 to 0.6) | .16 |
| Total NAFLD activity score (NAS) | |||||||||
| Patients with improvement | 66 (54%) | 52 (58%) | 14 (44%) | 1.8 (0.8 to 4.0) | .17 | 33 (53%) | 33 (55%) | 0.9 (0.5 to 1.9) | .84 |
| Patients with worse NAS | 30 (25%) | 20 (22%) | 10 (31%) | 0.6 (0.3 to 1.5) | .31 | 18 (29%) | 12 (20%) | 1.6 (0.7 to 3.8) | .25 |
| Change in score | −0.7 (1.9) | −0.8 (1.9) | −0.1 (1.1) | −0.5 (−1.1 to 0.2) | .17 | 1.7 (2.0) | −0.9 (1.7) | 0.4 (−0.2 to 1.0) | .16 |
| Hepatocellular ballooning | |||||||||
| Patients with improvement | 31 (25%) | 27 (30%) | 4 (12%) | 3.0 (1.0 to 9.4) | .06 | 22 (35%) | 9 (15%) | 3.1 (1.3 to 7.5) | .01 |
| Patients with worse ballooning | 19 (16%) | 12 (13%) | 7 (22%) | 0.6 (0.2 to 1.6) | .26 | 11 (18%) | 8 (13%) | 1.4 (0.5 to 3.8) | .50 |
| Change in score | −0.1 (0.8) | −0.2 (0.9) | 0.1 (0.7) | −0.3 (−0.6 to −0.1) | .02 | −0.2 (0.9) | −0.1 (0.7) | 0.1 (−1 to 0.4) | .28 |
| Steatosis | |||||||||
| Patients with improvement | 52 (43%) | 39 (43%) | 13 (41%) | 1.1 (0.5 to 2.5) | .79 | 23 (37%) | 29 (48%) | 0.6 (0.3 to 1.4) | .21 |
| Patients with worse steatosis | 19 (16%) | 13 (14%) | 6 (19%) | 0.7 (0.3 to 2.1) | .57 | 14 (23%) | 5 (8%) | 3.2 (1.1 to 9.6) | .04 |
| Change in score | −0.4 (1.0) | −0.5 (1.0) | −0.4 (1.1) | 0.0 (−4 to 0.4) | .98 | −0.2 (1.0) | −0.7 (1.0) | 0.2 (0.1 to 0.5) | .23 |
| Lobular inflammation | |||||||||
| Patients with improvement | 37 (30%) | 28 (31%) | 9 (28%) | 1.2 (0.5 to 2.8) | .75 | 18 (29%) | 19 (32%) | 0.9 (0.4 to 1.9) | .75 |
| Patients with worse lobular inflammation | 21 (17%) | 16 (18%) | 5 (16%) | 1.2 (0.3 to 3.5) | .78 | 12 (19%) | 9 (15%) | 1.4 (−0.5 to 3.5) | .53 |
| Change in score | −0.2 (0.8) | −0.2 (0.8) | −0.2 (0.8) | −0.2 (−4 to 0.1) | .20 | −0.1 (0.9) | −0.2 (0.7) | 0.1 (−0.1 to 0.3) | .40 |
| Portal inflammation | |||||||||
| Patients with improvement | 21 (17%) | 17 (19%) | 4 (12%) | 1.6 (0.5 to 5.27) | .41 | 14 (23%) | 7 (12%) | 2.2 (0.8 to 5.9) | .12 |
| Patients with worse portal inflammation | 21 (17%) | 15 (17%) | 6 (19%) | 0.9 (0.3 to 2.5) | .79 | 10 (16%) | 11 (18%) | 0.9 (0.3 to 2.2) | .75 |
| Change in score | −0.01 (0.61) | −0.02 (0.60) | 0.03 (0.65) | −0.2 ( −0.47 to 0.1) | .16 | −0.06 (0.62) | 0.05 (0.59) | −0.3 ( −0.5 to −0.1) | .007 |
Data are n (%) or mean ± SD.
Relative odds ratios (OR) and P values were calculated using logistic regression models of the change at end of follow-up for each characteristic on the subgroup (male vs female, adolescent vs preadolescent), for binary characteristics; P values and adjusted mean changes from baseline by each subgroup were calculated using analysis of covariance, regressing change from baseline to end of follow-up on each subgroup (either sex or age group) and the baseline value of the characteristic.
Categorized BMI z-score change from baseline has 3 categories, with gaining weight as the first category. Multinomial regression used to determine OR, 95% CI and P values, using ≥ 0 as the reference.
Diabetes at follow-up determined by case review using the definition of either a doctor-diagnosis by self-report or by a HbA1c at visit being ≥ 6.5%. MRI exam done only in CyNCh. 45 (32 males, 13 females; 23 <13 years, 22 13–17 years) children in the placebo group had both an enrollment and end of treatment examination.
Resolution of NASH was defined as a diagnosis of borderline or definite NASH at baseline and a diagnosis of not NALFD or NAFLD only at end-of-follow-up. Of the 96 patients with any NASH (borderline or definite at enrollment), 28 (29%) resolved to no NAFLD or NAFLD only at end of follow-up. 26 patients were excluded with NAFLD, no NASH at enrollment. Progression to definite NASH was defined as a NASH diagnosis of definite NASH at follow-up. There were 38 patients excluded from the analysis with definite NASH at baseline. Of the 84 children at risk of NASH progression, 15 (18%) progressed to definite NASH at end of follow-up. The patients with definite NASH at enrollment were distributed as: 29 male (32%), 9 female (28%); 29 adolescents (47%), 9 preadolescent (15%). Of the children at risk of progression to definite NASH: 61 male (73%), 23 female (27%); 33 adolescents (39%), 51 preadolescents (61%). Improvement in any histological feature was defined as a decrease at end of follow-up of 1 or more in the histological feature score. A patient was defined as having worse severity for a histological feature if there was an increase of 1 or more in the histological feature score.
No significant increases in mean glucose, HOMA-IR or HbA1C were observed between baseline and end-of-treatment. Overall, in the 122 participants, 8 (7%) had type 2 diabetes at baseline. By end-of-treatment, type 2 diabetes had developed in 6 (5%) additional participants, equivalent to an incidence of 29.3 cases/1000-person years (95% confidence interval [CI] 10.8–63.8 per 1000-person years). The proportion of new cases was higher in the adolescent age group but not statistically significant (8% vs 2%, P = .14). By end of follow-up, 2 additional children developed incident type 2 diabetes, resulting in a cumulative incidence rate of 36.8/1000-person years (95% CI 15.9–72.6/1000-person years).
We also compared the histology and laboratory outcomes and diabetes incidence separately for the shorter (CyNCh) and longer (TONIC) periods of observation for each placebo arm (Supplementary Table 1). There were no significant differences in the primary histologic outcomes of progression or resolution of NASH or fibrosis, or any improvement vs any progression in NASH and/or fibrosis. Likewise, there were no significant differences in changes in mean ALT, AST, GGT, fasting glucose, or HbA1c.
Regression in NAFLD Severity
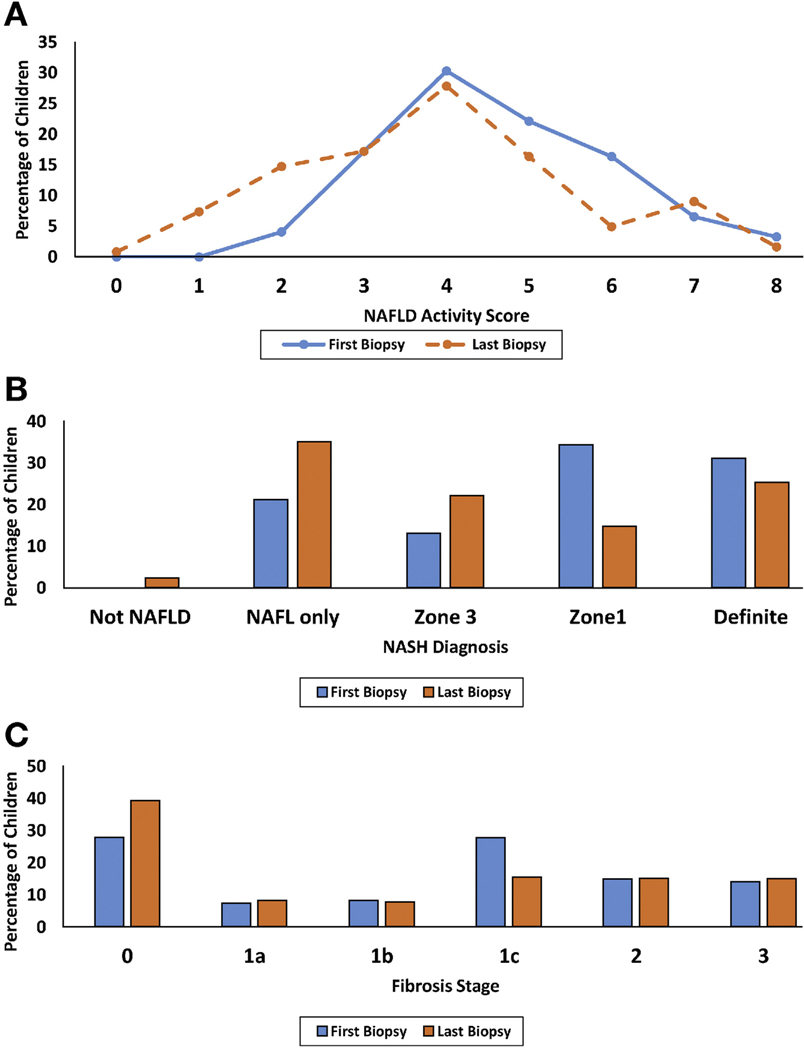
Table 2 shows changes in histological characteristics. Over an average 1.6 ± 0.4 years from baseline to end-of-treatment biopsy, 20% of the cohort achieved composite improvement with both resolution of NASH and fibrosis regression. Half (52%) demonstrated any improvement: resolution of NASH and/or regression of fibrosis. Of those with borderline or definite NASH at baseline, 29% had resolution of NASH. Of those with fibrosis at baseline, 34% improved. Half of the cohort (54%) showed improvement in total NAS and the mean composite NAFLD activity scores also declined over time from 4.6 ± 1.4 to 3.9 ± 1.7 (P = .001) (Figure 1A). Among the individual components of the NAS, steatosis improved in 43%, ballooning in 25%, lobular inflammation in 30%, and portal inflammation in 17%.
Figure 1.
Percentage of children with (A) NAFLD Activity Score, (B) NASH diagnosis, and (C) fibrosis stage, at first and last biopsy. First biopsy denoted in blue color and last biopsy denoted in orange color in each panel.
Collectively, the overall proportion of the cohort with NAFLD-not NASH increased (21% to 38%) and the proportion with definite NASH declined (31% to 25%) by end-of-treatment. However, only 3 participants (2.4%) resolved to not having NAFLD at follow-up; all had NAFLD-not NASH at baseline (Figure 1B). Likewise, the overall proportion with no fibrosis increased (28% to 39%) and mild fibrosis (stage 1a,1b,1c) declined (43% to 31%), while prevalence of stage 2 and stage 3 (bridging fibrosis) remained stable (14% to 15%, P = .03) (Figure 1C). No participant had cirrhosis at baseline (per protocol) and none progressed to cirrhosis by end-of-treatment.
Progression in NAFLD Severity
Composite progression to definite NASH and progression in fibrosis occurred in 11%, while any progression to definite NASH and/or fibrosis occurred in 36% of those with baseline NAFL or borderline NASH. Of those with baseline NAFL or borderline NASH, baseline, 18% progressed to definite NASH. Nearly one-quarter of the cohort (23%) progressed in fibrosis stage and a similar proportion (25%) experienced worsening in composite NAS. Among individual components of the NAS, steatosis worsened in 16%, ballooning in 16%, lobular inflammation in 17%, and portal inflammation in 17%.
Histological outcomes did not differ by sex; however, significant differences emerged by age group. Despite no significant difference in BMI z-score change, adolescents were more likely than preadolescents to experience improvement in ballooning (odds ratio [OR] 3.1; 95% CI 1.3–7.5; P = .01) yet more likely to develop worsening steatosis (23% vs 8%; OR 3.2; 95% CI 1.1–9.6; P = .04) and less likely to resolve NASH (17% vs 40%; OR 0.3; 95% CI 0.1–0.8; P = .02). Adolescents were also more likely to experience any progression to definite NASH and/or worsening fibrosis (OR 2.9; 95% CI 1.2–6.8; P = .02) and less likely to experience any resolution of NASH and/or regression in fibrosis stage (OR 0.5; 95% CI 0.2–1.0; P = .06).
In addition, the overall distribution of NASH phenotypes shifted over time (Figure 1B) to less pediatric-predominant borderline zone 1 NASH (34% to 15%) and more adult-predominant borderline zone 3 NASH (13% to 22%) (P < .0001). Of the 42 participants who had zone 1 borderline NASH pattern, about half (52%) transitioned to more adult phenotypes (36% borderline zone 3 and 17% definite NASH), while 38% resolved NASH over time and 10% maintained a pediatric periportal zone 1 pattern.
Clinical Characteristics Associated With Histological Progression vs Regression
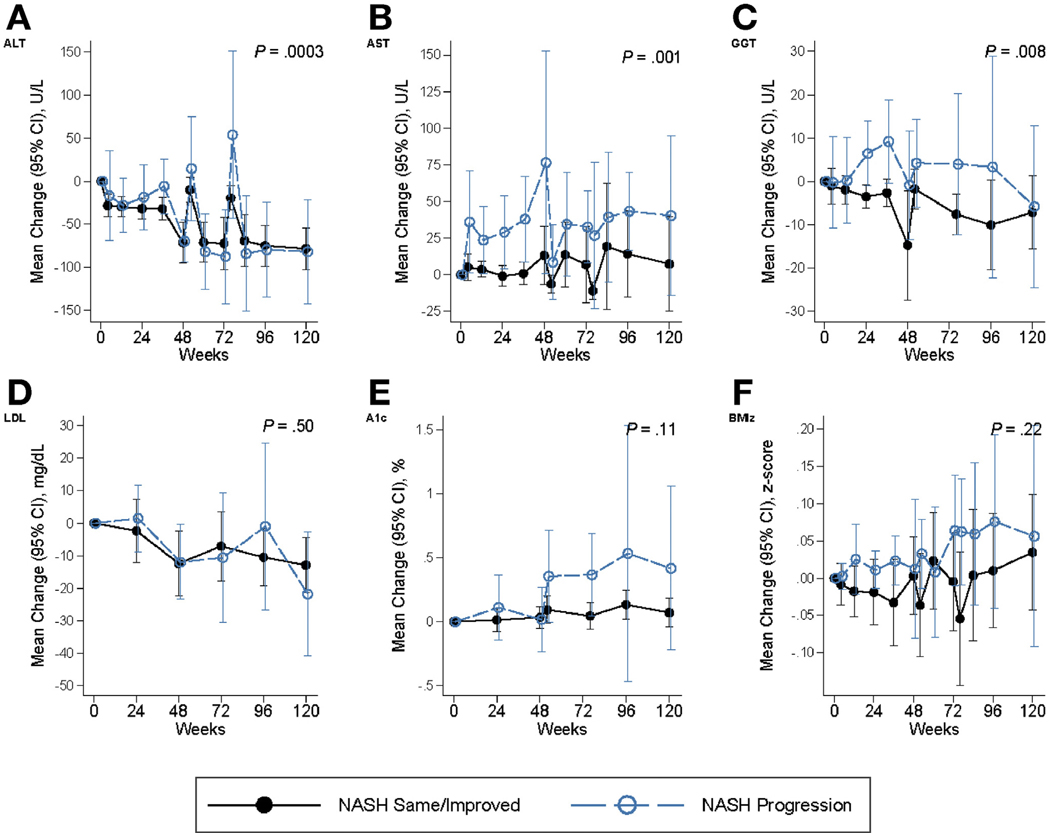
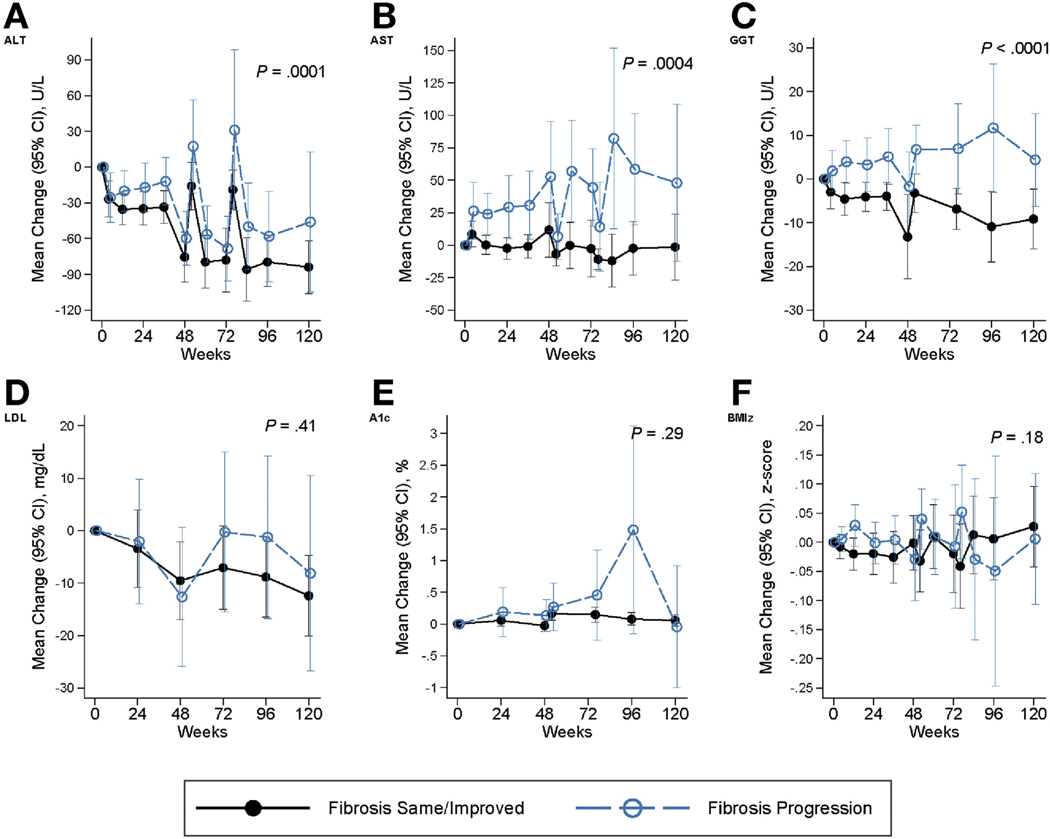
Figure 2 (combined placebo arms) and Supplementary Figure 2 (individual placebo arms) show mean changes over time in ALT, AST, GGT, HbA1C, LDL cholesterol, and BMI z-score in children with progression of NASH, compared to those with same or resolved NASH, and Figure 3 (combined placebo arms) and Supplementary Figure 3 (individual placebo arms) depict the change in the same variables over time in children with progression in fibrosis, compared to those with same or improved fibrosis. These measures generally worsened over time with progression compared to those without progression, with largest disparities for ALT, AST, and GGT (longitudinal P highly significant).
Figure 2.
Mean change from baseline in characteristics associated with progression vs same/improved NASH. Panels include (on y-axis) the following: (A) ALT (U/L), (B) AST (U/L), (C) GGT (U/L), (D) LDL cholesterol (mg/dL), (E) hemoglobin A1C (A1C) (%), and (F) BMI z-score (BMIz). The x-axis indicates the weeks of observation. Same/improved NASH is denoted by black solid lines, whereas progression in NASH is indicated by blue-dashed lines. Generalized estimating equations (GEEs) were used to account for repeated visits per child for multiple linear regression models of the change in laboratory test or BMI z-score in relation to the histological outcome indicator adjusted for the baseline value of the lab test or BMI z-score, the visit code and the interaction of visit by histological outcome, respectively; Probability (2-sided) then determined by testing the histological effect and the interaction term.
Figure 3.
Mean change from baseline in characteristics associated with progression vs same/improved fibrosis. Panels include (on y-axis): (A) ALT (U/L), (B) AST (U/L), (C) GGT (U/L), (D) LDL cholesterol (mg/dL), (E) hemoglobin A1C (A1C) (%), and (F) BMI z-score (BMIz). The x-axis indicates the weeks of observation. Same/improved fibrosis is denoted by black solid lines, while progression in fibrosis is indicated by blue-dashed lines. Generalized estimating equations (GEEs) were used to account for repeated visits for multiple regression models of the change in laboratory test or BMI z-score in relation to the histological outcome indicator adjusted for the baseline value of the laboratory test or BMI z-score, the visit code, and the interaction of visit by histological outcome, respectively. Probability (2-sided) then determined by testing the histological effect and the interaction term.
Resolution of NASH was associated with baseline younger age group, lower waist circumference, and ALT (P <.05), and with declining GGT (P < .01) and LDL cholesterol (P < .001) over time (Table 3). Greater reduction in BMI z-score associated with higher relative odds of NASH resolution (OR 2.08 for each decrease of −0.25 units, P = .04). Lower baseline AST and triglyceride levels approached significance for association with NASH resolution (P = .05). A decline in diastolic blood pressure percentile (P = .02) or GGT was associated with regression in fibrosis over time (P = .04, Table 3).
Table 3.
Participant characteristics associated with progression and improvement in NASH and fibrosis after follow-up in children with NAFLD
| Progression to Definite NASHa (n = 84) |
Resolution of NASHa (n = 96) |
Progression of Fibrosisa (n = 122) |
Fibrosis Improvementa (n = 122) |
|||||
|---|---|---|---|---|---|---|---|---|
| Characteristicsa | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb |
| Demographic: | ||||||||
| Gender: Female (vs male) | 0.96 (0.27–3.37) | .95 | 1.00 (0.36–2.77) | 1.00 | 0.92 (0.35–2.43) | .87 | 0.68 (0.28–1.63) | .38 |
| Age at randomization: 13–17 y (vs 8–12 y) | 1.45 (0.47–4.46) | .18 | 0.32 (0.12–0.82) | .02 | 2.05 (0.85–4.90) | .11 | 0.61 (0.29–1.30) | .20 |
| Ethnicity: Hispanic (vs non) | 1.31 (0.33–5.19) | .70 | 1.42 (0.50–4.05) | .51 | 0.81 (0.32–2.01) | .65 | 1.45 (0.62–3.41) | .39 |
| Race: White (vs Other) | 1.2 (0.37–3.93) | .75 | 1.02 (0.41–2.50) | .97 | 3.26 (1.14–9.33) | .03 | 0.64 (0.30–1.39) | .26 |
| Race/Ethnicity: | .95 | .44 | .48 | |||||
| Non-Hispanic White | 1.20 (0.34–4.29) | .82 | 0.58 (0.16–2.11) | .57 | 3.51 (1.04–11.88) | .043 | 0.55 (0.20–1.51) | .25 |
| Hispanic White | 1.21 (0.25–5.80) | .30 | 1.33 (0.50–3.52) | .57 | 3.12 (1.02–9.55) | .046 | 0.70 (0.30–1.63) | .41 |
| Other: any other race/ethnicity (reference category) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| PNPLA3 genotypec: n (%) | .68 | .41 | .74 | .42 | ||||
| CG | 0.54 (0.13–2.30) | .41 | 1.68 (0.61–4.63) | .31 | 0.69 (0.25–1.85) | .46 | 1.75 (0.74–4.15) | .21 |
| GG | 1.21 (0.11–13.25) | .88 | 0.48 (0.05–4.49) | .52 | 0.73 (0.14–3.98) | .72 | 0.97 (0.22–4.37) | .97 |
| CC (reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Baseline variables: | ||||||||
| Body Mass Index (BMI, kg/m2) | 1.08 (0.99–1.19) | .09 | 0.89 (0.81–0.98) | .02 | 1.04 (0.97–1.13) | .29 | 0.97 (0.90–1.04) | .37 |
| BMI z-score: | ||||||||
| ≥ 2.5 (severely obese) (vs<2.5) | 0.90 (0.22–3.61) | .12 | 0.45 (0.15–1.36) | .16 | 1.23 (0.48–3.18) | .66 | 0.58 (0.23–1.45) | .25 |
| BMI (for sex-age) percentile | 2.51 (1.00–6.29) | .05 | 0.85 (0.69–1.05) | .12 | 1.18 (0.91–1.54) | .21 | 1.01 (0.88–1.16) | .86 |
| Waist circumference (mm) | 1.37 (0.92–2.04) | .12 | 0.64 (0.45–0.90) | .01 | 1.02 (0.99–1.05) | .30 | 0.96 (0.74–1.25) | .79 |
| PDFF (%)c (n = 45) | 1.11 (1.0–1.22) | .05 | 0.95 (0.86–1.04) | .23 | 1.04 (0.97–1.12) | .23 | 1.01 (0.96–1.07) | .67 |
| Blood Pressure (BP) | ||||||||
| Systolic blood pressure (mm Hg) | 1.02 (0.98–1.07) | .31 | 0.98 (0.95–1.01) | .19 | 1.01 (0.98–1.05) | .37 | 1.00 (0.97–1.02) | .84 |
| Diastolic blood pressure (mm Hg) | 1.05 (0.99–1.11) | .08 | 9.96 (0.91–1.01) | .15 | 1.02 (0.97–1.06) | .46 | 1.00 (0.97–1.04) | .82 |
| Blood pressure percentiles: | ||||||||
| Systolic BP (adj sex-age-ht) | 1.01 (0.99–1.04) | .28 | 0.99 (0.98–1.01) | .55 | 1.01 (0.99–1.02) | .52 | 1.00 (0.98–1.01) | .84 |
| Systolic BP ≥ 95%ile | 1.85 (0.58–5.94) | .30 | 1.20 (0.46–3.12) | .71 | 0.77 (0.30–2.03) | .60 | 1.22 (0.54–2.78) | .63 |
| Diastolic BP (adj sex-age-ht) | 1.02 (1.00–1.04) | .09 | 0.99 (0.97–1.01) | .26 | 1.00 (0.98–1.02) | .96 | 1.01 (0.99–1.02) | .42 |
| Diastolic BP ≥ 95%ile | 5.42 (0.98–30.09) | .05 | 0.62 (0.07–5.77) | .67 | 3.71 (0.86–15.92) | .08 | 1.18 (0.27–5.22) | .82 |
| Diabetes | ||||||||
| Diabetes type 2 (diagnosed or A1c≥ 6.5% (n = 8) | 1.57 (0.15–16.24) | .70 | 0.79 (0.15–4.20) | .79 | 0.46 (0.05–3.91) | .48 | 2.0 (0.47–8.44) | .34 |
| Laboratory: | ||||||||
| ALT (per 10 U/L change) | 3.24 (1.32–7.92) | .01 | 0.39 (0.17–0.93) | .03 | 1.18 (0.67–2.08) | .57 | 0.85 (0.49–1.48) | .58 |
| AST (per 10 U/L change) | 6.56 (1.30–33.0) | .02 | 0.21 (0.04–0.99) | .05 | 1.17 (0.39–3.45) | .78 | 0.91 (0.33–2.46) | .85 |
| GGT (per 10 U/L change) | 6.05 (1.20–30.6) | .03 | 0.18 (0.03–1.29) | .09 | 0.58 (0.12–2.77) | .50 | 0.82 (0.23–2.88) | .75 |
| Total cholesterol (mg/mL) | 5.23 (1.10–25.0) | .04 | 0.26 (0.06–1.09) | .07 | 2.44 (0.60–9.94) | .21 | 0.39 (0.13–1.17) | .09 |
| HDL cholesterol (mg/mL) | 0.79 (0.00–280) | .94 | 10.6 (0.16–697) | .27 | 0.16 (0.0–18.3) | .44 | 1.84 (0.04–93) | .76 |
| LDL cholesterol (mg/mL) | 7.79 (1.19–50.93) | .03 | 0.24 (0.05–1.22) | .09 | 2.44 (0.60–9.94) | .21 | 0.35 (0.10–1.26) | .11 |
| Triglycerides (mg/mL) | 1.10 (0.53–2.30) | .79 | 0.48 (0.24–0.99) | .05 | 1.26 (0.75–2.13) | .39 | 0.77 (0.46–1.28) | .32 |
| HOMA-IR/10d | 1.71 (0.70–4.20) | .24 | 0.75 (0.41–1.39) | .37 | 0.95 (0.66–1.38) | .80 | 1.15 (0.85–1.55) | .37 |
| HbA1c (%) | 3.84 (0.78–19.0) | .10 | 0.57 (0.16–2.02) | .38 | 1.23 (0.41–3.69) | .71 | 1.01 (0.38–2.70) | .98 |
| Quality of Life (QOL) | ||||||||
| Self-reported pediatric QOL | ||||||||
| Physical health | 1.00 (0.97–1.03) | .92 | 1.02 (0.99–1.04) | .23 | 0.995 (0.97–1.02) | .65 | 0.99 (0.97–1.01) | .27 |
| Psychosocial health | 0.98 (0.95–1.02) | .31 | 1.00 (0.98–1.03) | .82 | 0.995 (0.97–1.02) | .67 | 0.98 (0.96–1.00) | .06 |
| Parent/Guardian-reported pediatric QOL | ||||||||
| Physical health | 1.01 (0.98–1.02) | .94 | 1.01 (0.99–1.02) | .56 | 0.996 (0.98–1.01) | .67 | 0.99 (0.98–1.01) | .32 |
| Psychosocial health | 1.01 (0.98–1.04) | .49 | 1.02 (1.00–1.04) | .09 | 1.002 (0.98–1.02) | .84 | 0.98 (0.96–0.99) | .04 |
| Change from baseline at 52 or 96 weeks: | ||||||||
| Body Mass Index (BMI, kg/m2) | 1.19 (0.89–1.59) | .25 | 0.86 (0.71–1.05) | .14 | 0.99 (0.84–1.16) | .88 | 0.997 (0.86–1.16) | .97 |
| BMI z-score mean change | 7.70 (0.30–198) | .22 | 0.23 (0.02–2.14) | .20 | 1.59 (0.19–13.1) | .66 | 1.06 (0.17–6.56) | .95 |
| BMI z-score (3-category)d | 0.29 (0.8–1.02) | .05 | 2.08 (1.02–4.27) | .04 | 0.64 (0.31,1.31) | .22 | 1.09 (0.61–1.96) | .76 |
| Reference (≥ 0 higher BMIZ) | ||||||||
| BMI (for sex-age) %tile | 1.12 (0.83–1.52) | .45 | 1.15 (0.86–1.54) | .35 | 1.10 (0.87–1.39) | .43 | 1.10 (0.90–1.34) | .36 |
| PDFF (%)c (n = 45) | 0.97 (0.88–1.08) | .60 | 0.96 (0.88–1.06) | .43 | 0.992 (0.89–1.10) | .88 | 1.01 (0.94–1.09) | .81 |
| Blood Pressure (BP): | ||||||||
| Systolic blood pressure (mm Hg) | 1.02 (0.98–1.06) | .43 | 1.00 (0.96–1.03) | .95 | 1.00 (0.96–1.03) | .37 | 0.99 (0.97–1.02) | .73 |
| Diastolic blood pressure (mm Hg) | 1.02 (0.97–1.09) | .41 | 0.97 (0.92–1.02) | .25 | 1.03 (0.98–1.07) | .30 | 0.95 (0.91–0.99) | .02 |
| Blood pressure %tiles: | ||||||||
| Systolic BP (adj sex-age-ht) | 1.01 (1.00–1.03) | .14 | 0.99 (0.78–1.26) | .97 | 1.003 (0.99–1.02) | .65 | 0.996 (0.98–1.01) | .59 |
| Diastolic BP (adj sex-age-ht) | 1.01 (0.99–1.03) | .21 | 1.00 (0.98–1.02) | 1.00 | 1.01 (0.99–1.02) | .27 | 0.98 (0.97–1.00) | .03 |
| Diabetes type 2: | ||||||||
| Incident cases (n = 6)b | 4.86 (0.29–82.38) | .27 | 0.34 (0–2.63)† | .34 | 32.91 (4.54–N)† | .0002 | 0.22 (0–1.58)† | .15 |
| Diabetes at any visit (n = 14) | 2.50 (0.41–15.11 | .32 | 0.40 (0.08–1.93) | .25 | 4.14 (1.31–13.1) | .02 | 0.74 (0.22–2.51) | .63 |
| Quality of Life (QOL) | ||||||||
| Self-reported pediatric QOL | ||||||||
| Physical health | 0.96 (0.92–1.00) | .03 | 0.99 (0.97–1.02) | .62 | 0.98 (0.96–1.00) | .11 | 1.02 (0.99–1.04) | .16 |
| Psychosocial health | 0.96 (0.93–1.00) | .06 | 1.00 (0.98–1.03) | .72 | 0.99 (0.96–1.01) | .27 | 1.03 (1.00–1.05) | .046 |
| Parent/Guardian-reported pediatric QOL | ||||||||
| Physical health | 1.00 (0.98–1.03) | .75 | 1.00 (0.98–1.02) | .76 | 0.99 (0.97–1.01) | .41 | 1.01 (0.99–1.03) | .31 |
| Psychosocial health | 1.00 (0.98–1.03) | .92 | 0.9 (0.96–1.00) | .12 | 0.99 (0.97–1.01) | .54 | 1.01 (0.99–1.03) | .22 |
| Laboratory: | ||||||||
| Change in ALT (per 10 U/L change) | 1.63 (0.68–3.95) | .28 | 0.67 (0.39–1.15) | .15 | 2.40 (1.20–4.81) | .01 | 0.68 (0.42–1.13) | .14 |
| Change in AST (per 10 U/L change) | 3.74 (0.49–28.58) | .20 | 0.45 (0.16–1.27) | .13 | 3.57 (0.97–13.1) | .06 | 0.58 (0.23–1.49) | .26 |
| Change in GGT (per 10 U/L change) | 11.04 (0.41–296) | .15 | 0.04 (0.00–0.40) | .007 | 83.4 (5.4–1289) | .002 | 0.13 (0.02–0.94) | .04 |
| Change in HDL cholesterol (mg/mL) | 0.02 (0.00–27.71) | .28 | 0.79 (0.00,236) | .94 | 0.99 (0.93–1.05) | .67 | 0.99 (0.94–1.04) | .63 |
| Change in LDL cholesterol (mg/mL) | 2.73 (0.16–46.31) | .49 | 0.24 (0.23–0.59) | <.001 | 1.80 (0.23–13.9) | .57 | 0.98 (0.17,5.80) | .99 |
| Change in triglycerides (mg/mL) | 1.69 (0.73–3.93) | .22 | 0.83 (0.40–1.72) | .61 | 1.50 (0.78–2.87) | .22 | 0.82 (0.46–1.46) | .51 |
| Change in HOMA-IR/100e | 2.53 (0.09–71.2) | .59 | 2.46 (0.21–29.7) | .48 | 2.30 (0.17–31.3) | .53 | 0.21 (0.02–2.80) | .23 |
| Change in HbA1c (%) | 3.36 (0.99–11.42) | .05 | 0.45 (0.28–0.72) | .16 | 2.27 ( 1.10–4.67) | .03 | 0.57 (0.25,1.33) | .19 |
Total number of TONIC and CyNCH participants assigned to Placebo group with paired baseline and end-of-treatment biopsies is 122. Progression to definite NASH was defined as a NASH diagnosis at follow-up of being a definite NASH. Any patient diagnosed as definite NASH at baseline were excluded from the analysis. Of the 84 children at risk of NASH progression, 15 (18%) progressed to definite NASH at end of follow-up; 38 children with definite NASH at enrollment were excluded. Resolution of nonalcoholic steatohepatitis (NASH) was defined as a diagnosis of borderline or definite NASH at baseline and a diagnosis of not NAFLD or NAFLD only at end-of-follow-up. Of the 96 patients with NASH (borderline or definite at enrollment), 28 (29%) resolved to no NAFLD or NAFLD only at end of follow-up. 26 patients were excluded with NAFLD, no NASH at enrollment. Progression of fibrosis defined as an increase of 1 or more stage in fibrosis at end of follow-up from baseline. All children had the potential to progress. 28/122 (23%) of the children had fibrosis progression. Improvement in fibrosis defined as a decrease of 1 or more stage in fibrosis at end of follow-up from baseline. 42/122 (34%) of the children had improvement in fibrosis.
P (2-sided) determined from logistic regression models of the histological outcome on each characteristic. An exact logistic regression was used to analyze incident diabetes for the outcomes resolution of NASH (no patients with incident diabetes resolved), progression to fibrosis (all patients with incident diabetes had fibrosis progression) and fibrosis improvement (all patients with diabetes did not improve).
25/122 (20%) of the children were not genotyped. MRI exam done only in CyNCh. 45 children in the placebo group had both an enrollment and end of treatment exam.
BMI z-score change from baseline (3-category) is coded as 0=higher BMI z-score, 1 =decrease by >0 to −0.25, 2=decrease by ≥ −0.25 BMI z-score. Logistic regression used to determine OR, 95% CI, P-value, in which BMI z-score change is analyzed as a linear trend. Interpretation is that the odds of the outcome increases (or decreases) per unit increase in BMI z-score change.
HOMA-IR units are mg/dL x μU/mL/405
Progression to definite NASH was associated with higher baseline ALT, AST, GGT, total and LDL cholesterol levels, and increasing BMI z-score (Table 3, all P < .05). Worsening HbA1C also approached significance in those with progression to NASH, with an RR of 3.4 (95% CI 0.99–11.4; P = .05). Progression in fibrosis associated with white race (similarly for Hispanic-white and non-Hispanic-white ethnicity), rise in ALT, GGT, and HbA1C over time, and incident type 2 diabetes (P < .05 for all). The highest relative odds were associated with development of type 2 diabetes (OR 32.9; 95% CI 4.5– ∞ ; P = .0002) and rising GGT level (OR 83.4; 95% CI 5.4–1289; P = .002).
Any progression (in fibrosis and/or to definite NASH) was associated with adolescent age, higher waist circumference, ALT, AST, total and LDL cholesterol, as well as increasing ALT, HbA1C, and particularly GGT (Table 4). Not only was rising GGT the strongest risk factor for any progression (OR 45.4; 95% CI 2.59–797; P = .009), it was the only measure associated with lower odds of experiencing any improvement in fibrosis regression and/or NASH resolution (OR 0.03; 95% CI 0.002–0.29; P = .003). Having type 2 diabetes at any visit, as well as incident type 2 diabetes, were also associated with substantially increased odds of any progression (both P < .01). Hispanic ethnicity did not associate with any progression or any improvement, including when further categorized by white or other race.
Table 4.
Participant characteristics associated with any progression or any improvement in fibrosis and/or NASH after follow-up in children with NAFLD
| Any Progression in: Fibrosis or NASHa (n = 95) |
Any Improvement in: Fibrosis or NASHa (n = 99) |
|||
|---|---|---|---|---|
| Characteristicsa | OR (95% CI) | Pb | OR (95% CI) | Pb |
| Demographic: | ||||
| Gender: Female (vs male) | 1.01 (0.39–2.62) | .98 | 1.03 (0.41–2.54) | .96 |
| Age at randomization: | ||||
| 13 – 17 y (vs 8–12 y) | 2.86 (1.20–6.81) | .02 | 0.46 (0.21–1.03) | .06 |
| Ethnicity: Hispanic (vs non) | 0.78 (0.31–2.00) | .61 | 1.82 (0.74–4.46) | .19 |
| Race: White (vs Other) | 1.80 (0.72–4.52) | .21 | 0.73 (0.35–1.77) | .56 |
| Race/Ethnicity: | .32 | .19 | ||
| Non-Hispanic White | 2.42 (0.79–7.65) | .13 | 0.43 (0.15–1.26) | .12 |
| Hispanic White | 1.54 (0.57–4.16) | .40 | 1.11 (0.45–2.76) | .82 |
| Other: any other race/ethnicity (reference category) | 1.00 | 1.00 | ||
| PNPLA3 genotypec: n (%) | 0.78 | .08 | ||
| CG | 0.78 (0.29–2.11) | .63 | 2.78 (1.06–7.26) | .04 |
| GG | 1.41 (0.25–7.86) | .69 | 0.76 (0.16–3.58) | .73 |
| CC (reference) | 1.00 | 1.00 | ||
| Baseline variables: | ||||
| Body Mass Index (BMI, kg/m2) | 1.09 (1.01–1.18) | .03 | 0.97 (0.90–1.04) | .37 |
| BMI z-score: | .71 | .20 | ||
| ≥ 2.5 (severely obese) (vs. <2.5) | 1.21 (0.50–3.18) | 0.56 (0.23–1.35) | ||
| BMI (for age) percentile | 1.36 (0.99–1.87) | .06 | 0.96 (0.79–1.16) | .64 |
| Waist circumference (mm) | 1.48 (1.08–2.01) | .01 | 0.83 (0.63–1.10) | .20 |
| PDFF (%)c (n = 85) | 1.06 (0.99–1.14) | .08 | 0.96 (0.89–1.04) | .29 |
| Blood Pressure (BP): | ||||
| Systolic blood pressure (mmHg) | 1.02 (0.99–1.05) | .22 | 1.00 (0.96–1.02) | .49 |
| Diastolic blood pressure (mmHg) | 1.03 (0.98–1.07) | .27 | 1.01 (0.96–1.05) | .81 |
| Blood pressure percentiles: | ||||
| Systolic BP (adj sex-age-ht) | 1.006 (0.99–1.02) | .49 | 1.00 (0.98–1.01) | .63 |
| Systolic BP ≥ 95%ile | 0.91 (0.35–2.35) | .85 | 1.14 (0.84–2.70) | .76 |
| Diastolic BP (adj sex-age-ht) | 1.004 (0.99–1.02) | .62 | 1.004 (0.99–1.02) | .61 |
| Diastolic BP ≥ 95%ile | 3.87 (0.667–22.3) | .13 | 2.00 (0.35–11.46) | .44 |
| Diabetes | ||||
| Diabetes type 2 (diagnosed or A1c≥ 6.5% (n = 8) | 1.84 (0.25–13.71) | .55 | 1.63 (0.37–7.23) | .52 |
| Laboratory: | ||||
| ALT (per 10 U/L change) | 2.28 (1.10–4.70) | .03 | 0.70 (0.39–1.24) | .22 |
| AST (per 10 U/L change) | 4.15 (1.08–16.02) | .04 | 0.57 (0.21–1.60) | .29 |
| GGT (per 10 U/L change) | 2.81 (0.69–11.41) | .15 | 0.56 (0.16–1.97) | .37 |
| Total cholesterol (mg/mL) | 3.63 (1.11–11.91) | .03 | 0.41 (0.12–1.37) | .15 |
| HDL cholesterol (mg/mL) | 0.08 (0.00–9.16) | .29 | 6.70 (0.13–358) | .35 |
| LDL cholesterol (mg/mL) | 4.08 (1.00–16.68) | .05 | 0.34 (0.08–1.40) | .14 |
| Triglycerides (mg/mL) | 1.43 (0.84–2.45) | .18 | 0.69 (0.40–1.19) | .18 |
| HOMA-IR/10d | 1.51 (0.77–2.96) | .23 | 1.10 (0.80–1.52) | .54 |
| HbA1c (%) | 2.91 (0.80–10.50) | .11 | 0.88 (0.31–2.48) | .80 |
| Quality of Life (QOL) | ||||
| Self-reported pediatric QOL | ||||
| Physical health | 0.997 (0.98–1.02) | .75 | 1.00 (0.98–1.02) | .98 |
| Psychosocial health | 0.997 (0.97–1.02) | .80 | 0.99 (0.97–1.01) | .47 |
| Parent/Guardian-reported pediatric QOL | ||||
| Physical health | 0.99 (0.97–1.0) | .46 | 1.00 (0.98–1.02) | .93 |
| Psychosocial health | 1.001 (0.98–1.02) | .94 | 0.99 (0.97–1.01) | .57 |
| Change from baseline at 52 or 96 weeks: | ||||
| Body Mass Index (BMI, kg/m2) | 1.02 (0.86–1.22) | .80 | 0.97 (0.82–1.14) | .72 |
| BMI z-score mean change | 1.98 (0.26–15.29) | .51 | 0.72 (0.9–5.45) | .75 |
| BMI z-score (3-category)d | 0.55 (0.26–1.14) | .11 | 1.20 (0.63–2.27) | .58 |
| Reference (≥ 0 higher BMIz) | ||||
| PDFF (%)c (n = 45) | 0.96 (0.88–1.05) | .41 | 1.00 (0.93–1.08) | .92 |
| Blood Pressure (BP): | ||||
| Systolic blood pressure (mmHg) | 1.03 (0.98–1.06) | .07 | 0.994 (0.97–1.02) | .68 |
| Diastolic blood pressure (mmHg) | 1.07 (1.01–1.03) | .02 | 0.95 (0.90–1.00) | .048 |
| Blood Pressure percentiles: | ||||
| Systolic BP (adj sex-age-ht) | 1.01 (0.996–1.02) | .14 | 1.00 (0.99–1.02) | .77 |
| Diastolic BP (adj sex-age-ht) | 1.01 (0.99–1.02) | .28 | 0.99 (0.97–1.00) | .10 |
| Diabetes type 2: | ||||
| Incident casesc (n = 6) | 16.95 (2.34–N) | .003 | 0.54 (0–7.10) | .65 |
| Diabetes at any visit (n = 14) | 9.08 (1.80–45.72) | .007 | 0.54 (0.16–1.80) | .32 |
| Quality of Life (QOL) | ||||
| Self-reported pediatric QOL | ||||
| Physical health | 0.98 (0.96–1.00) | .10 | 1.02 (1.00–1.04) | .13 |
| Psychosocial health | 0.98 (0.95–1.01) | .11 | 1.02 (0.99–1.04) | .12 |
| Parent/Guardian-reported pediatric QOL | ||||
| Physical health | 0.997 (0.98–1.02) | .75 | 1.01 (1.00–1.03) | .11 |
| Psychosocial health | 1.00 (0.98–1.02) | .84 | 1.01 (0.99–1.02) | .53 |
| Laboratory: | ||||
| Change in ALT (per 10 U/L change) | 2.31 (1.13–4.75) | .02 | 0.58 (0.33–1.01) | .06 |
| Change in AST (per 10 U/L change) | 3.93 (0.94–16.45) | .06 | 0.44 (0.16–1.23) | .12 |
| Change in GGT ((per 10 U/L change) | 45.4 (2.59–797) | .009 | 0.03 (0.002–0.29) | .003 |
| Change in HDL cholesterol(mg/mL) | 0.98 (0.93–1.04) | .52 | 0.99 (0.94–104) | .70 |
| Change in LDL cholesterol (mg/mL) | 1.70 (0.22–13.05) | .61 | 0.78 (0.13–4.64) | .78 |
| Change in triglycerides (mg/mL) | 1.64 (0.85–3.12) | .14 | 0.66 (0.34–1.29) | .23 |
| Change in HOMA-IR/10e | 3.08 (0.17–57.44) | .45 | 0.45 (0.05–4.25) | .49 |
| Change in HbA1c (%) | 2.58 (1.05–6.30) | .04 | 0.58 (0.29–1.19) | .14 |
Total number of TONIC and CyNCh participants assigned to Placebo group with paired baseline and end-of-treatment biopsies is 122. Progression to NASH or fibrosis was defined as either a NASH diagnosis at follow-up of being definite NASH, or having an increase of 1 or more stage in fibrosis at end of follow-up. Patients diagnosed as definite NASH at baseline without progression of fibrosis were excluded from the analysis (27 children were excluded from the analysis); 34/95 (36%) had progression in either endpoint. Improvement in fibrosis or NASH was defined as either: a diagnosis of borderline or definite NASH at baseline and a diagnosis of not NALFD or NAFLD only at end-of-follow-up, or an increase of 1 or more stage in fibrosis at end of follow-up. Patients diagnosed as NAFLD, not NASH at baseline without improvement in fibrosis were excluded from the analysis (23 children were excluded); 51/99 (52%) had improvement in either endpoint.
P (2-sided) determined from logistic regression models of the histological outcome on each characteristic. An exact logistic regression was used to analyze diabetes incidence for both outcomes, as no patient with diabetes at follow-up improved and all progressed in disease.
25/122 (20%) of the children were not genotyped. MRI exam done only in CyNCh. 45 children in the placebo group had both an enrollment and end of treatment exam.
BMI z-score change from baseline (3-category) is coded as 0 =higher BMI z-score, 1=decrease by >0 to −0.25, 2=decrease by ≥ −0.25 BMI z-score. Logistic regression used to determine OR, 95% C.I., P, in which BMI z-score change is analyzed as a linear trend.
HOMA-IR units are mg/dL x μU/mL/405
PNPLA3 CG genotype associated positively with any improvement in NASH and/or fibrosis (OR 2.78; 95% CI 1.06–7.26; P = .04) compared with CC genotype. However, GG genotype did not demonstrate any significant associations.
Discussion
Receiving standard-of-care lifestyle advice over a 1- or 2-year period was associated with resolution of borderline or definite NASH in nearly one-third of children in the placebo arms of 2 multicenter NASH CRN clinical trials. Further, one-third also demonstrated regression in fibrosis. In total, half the cohort demonstrated improvement in fibrosis and/or NASH resolution. However, only 3 children completely resolved NAFLD. The long-term durability of improvement remains unknown. Notably, more than one-third of children progressed to definite NASH and/or worsening fibrosis over time, despite standard of care lifestyle advice provided every 3 months, highlighting the insufficiency of this first-line approach for NAFLD in a substantial proportion of subjects.
Widely available, easily measured clinical risk factors emerged that associated with risk of disease progression or regression. Greater decrease in BMI z-score proportionally reduced odds of progression to definite NASH, compared with no change or worsening BMI z-score, but was not associated with change in fibrosis. Although higher baseline ALT, AST, GGT were associated with progression to definite NASH, further increases in serum aminotransferase levels over time were not. However, rising ALT, HbA1c, and GGT were associated with progression in fibrosis, as well as any progression (to definite NASH and/or in fibrosis). Increasing GGT and having or developing type 2 diabetes were the strongest risk factors for progression in fibrosis or any progression, whereas rising GGT was the only factor associated with reduced odds of any improvement (NASH resolution and/or fibrosis regression). Recognizing these risk factors may enhance identification of children not responding to first-line lifestyle counseling and in need of more intensive interventions.
Only 2 longitudinal studies have retrospectively analyzed serial liver biopsies from children with NAFLD but sample sizes were very small. In a single-center cohort of 106 children with biopsy-confirmed NAFLD with mean age 13.4 years, range 4 to 18 years, 18 had a follow-up liver biopsy at a mean of 28 months from initial biopsy.12 Seven of these patients had no change in fibrosis, whereas 8 had worsening of fibrosis and only 3 achieved improvement in fibrosis. The small sample size precluded analyzing predictors of histological outcomes. A second single-center study retrospectively analyzed outcomes of 66 children with biopsy-confirmed NAFLD, mean age 13.9 years, for up to 20 years, but only 13 serial liver biopsies were obtained in 5 children over a mean of 41 months.9 Fibrosis progressed in 4 of the 5. In the entire cohort, 2 children died and 2 underwent liver transplantation for decompensated cirrhosis.
Although fibrosis progressed in nearly a quarter, the overall prevalence of bridging fibrosis remained unchanged at 15% and no one progressed to cirrhosis over the course of study. However, longer-term studies are indicated to monitor these children and determine the rate of progression to cirrhosis and end-stage liver disease as they become young adults. Other single-center case reports have documented that some children with NASH can rapidly progress to advanced fibrosis over a period of 1 to 2 years; most of these children were peripubertal or post-pubertal.10–12 Although still uncommon, there has been a progressive increase in NASH-related cirrhosis as the primary indication for liver transplantation in children and young adults between 2001 and 2012.18 Of 330 children and young adult patients (<40 years of age) undergoing liver transplantation in the United States for NASH-related cirrhosis from 1987 to 2012, 4% were younger than 18 and 16% were 18 to 29 years old.18 Many of the younger adults likely had unrecognized NAFLD as children.
In comparison, approximately 25% of adults with NASH are estimated to progress to advanced fibrosis/cirrhosis over 10 years.19 A systematic review and meta-analysis of studies including paired liver biopsies found that 36% of 311 adults with NAFLD developed progressive fibrosis.20 Risk factors for progression included hypertension and low AST/ALT ratio at baseline; others studies have reported older age and inflammation on initial biopsy as risk factors for fibrosis progression.21 NASH can rapidly progress to advanced fibrosis over a mean of 5.9 years in some adults.20 NASH also carries increased risk of cardiovascular disease, and liver-related and all-cause mortality in adults.22–25 NASH-related cirrhosis is now the second leading indication for liver transplantation in adults and is projected to become the leading cause within the next decade.26,27
Children who develop NAFLD and NASH early in life may have different genetic and/or environmental propensities for the disease compared with those with onset in adulthood. The development of NAFLD in childhood may worsen long-term morbidity and mortality, given the anticipated longer disease duration and sustained exposure to the proinflammatory milieu of obesity and associated cardiometabolic risk factors. This hypothesis is supported by the association we found between worsening obesity and increased risk of progression to NASH, and rising HbA1c and incident type 2 diabetes as risk factors for fibrosis progression. Moreover, the high incidence of type 2 diabetes we observed in these children with NAFLD, nearly 300-fold the reported incidence rate in the general pediatric population age 10 to 19 years old, is a significant concern and supports recommendations to screen for diabetes development in children with NAFLD.8,28 Although white race, both of Hispanic and non-Hispanic ethnicity, was associated with higher relative risk of fibrosis progression, the children of Hispanic ethnicity were younger in age, suggesting increased susceptibility. We also observed a shift from the more periportal patterns of histological NASH common in children to more adult phenotypes of definite NASH and borderline zone 3 NASH, although a periportal-predominant pattern persisted in some children. Whether persistence of periportal disease confers unique clinical or histological outcomes with increasing age requires further study, but some studies report higher rates of fibrosis in adults with periportal patterns of disease.29
Our study highlights the benefits and limitations of standard lifestyle counseling and the need to develop more effective treatments for pediatric NAFLD. Although improvement in BMI z-score was proportionally associated with resolution of NASH, more than half of the cohort developed worsening obesity, which was significantly associated with progression to NASH. For children failing to respond to standard lifestyle counseling, multidisciplinary interventions can be more effective, particularly with increased contact hours and additional behavioral counseling.15,30 However, these resource-intensive multidisciplinary programs are less available, affordable, or accessible. They are also largely ineffective for severely obese children, who comprised a substantial proportion of our cohort (25% with BMI z-score ≥2.5).31 Bariatric surgery can benefit severely obese children with NASH and other severe comorbidities, including type 2 diabetes, but is not widely scalable or necessarily appropriate for all children with NASH.32 Pharmacotherapy for pediatric NASH is lacking. Whereas supplemental vitamin E can lead to NASH resolution in some children and adults, further studies are needed to demonstrate sustained benefit, optimal dose, and long-term safety.8,13
This study is the first prospective study of the natural history of NAFLD in children using per-protocol, timed liver biopsy reassessment in children enrolled in the placebo arms of 2 large multicenter clinical trials. These children received only standard-of-care lifestyle advice aligned with guidance that would be provided in routine primary care of any child with overweight or obesity. Identifying demographic and clinical characteristics associated with disease progression vs regression in this population is both novel and clinically relevant, and the vast majority (88%) completed the trial in entirety. Additional strengths include ethnic and racial diversity, double-blind collection of detailed anthropometric and clinical data, including central scoring of the paired liver biopsies by a committee of pathologists with expertise in NAFLD. In comparison with adults, these children have onset of disease early in life, which may reflect genetic or environmental vulnerabilities to development of NAFLD, and likely translate to higher longer-term risks. Although PNPLA3 genotype had very limited association with histological change in this cohort, genotype data were not available in 20% of the cohort, resulting in reduced power to determine significance. Other limitations of this study include the relatively short duration that precludes determining outcomes on mortality, cardiovascular disease, diabetes incidence, and quality of life. In addition, participating in a placebo arm could introduce selection bias or bias results toward more favorable outcomes due to placebo effect. A larger sample size would further validate significant risk factors and estimates of associated risks. Last, liver biopsy remains the gold standard for evaluating severity of NAFLD, but sampling variability can occur.33 Existing noninvasive serological tests for detecting advanced fibrosis have not been demonstrated to be sufficiently accurate in children with NAFLD, highlighting the need to improve noninvasive assessments of NAFLD in children.34 Magnetic resonance imaging assessments of hepatic steatosis and stiffness have shown promise in cross-sectional analyses in children with NAFLD and may be valuable in future natural history studies in children.35–37
In conclusion, half of the children exhibited some improvement in NASH or fibrosis severity with standard-of-care lifestyle counseling in our prospective placebo cohorts; however, ongoing progression occurred in more than one-third within 2 years. Progression to NASH and/or worsening fibrosis was associated with adolescent age, higher baseline central adiposity, serum aminotransferase, total and LDL cholesterol levels, and worsening ALT, GGT, and HbA1c over time, which will help identify children at highest risk. The high incidence of type 2 diabetes, and its strong relationship to fibrosis progression, underscores the urgent need to assess longer-term outcomes. Trials of novel therapies are urgently needed to identify efficacious and accessible interventions for children failing to respond to lifestyle counseling.38
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
Little is known about clinical and histological outcomes of children with fatty liver disease.
NEW FINDINGS
A study of 139 children who received only standard of care lifestyle advice and placebo in 2 double-blind, randomized clinical trials found that, over 2 years, features of fatty liver decreased in approximately half of the children, but histologic features of disease severity increased in approximately one-third and 5% developed type 2 diabetes. Complete resolution of NAFLD was rare.
LIMITATIONS
The children in this study all received placebo and standard of care lifestyle advice while they were participants in clinical trials. It is unclear whether these findings apply to children with fatty liver disease in primary care or in the general population.
IMPACT
Although standard of care lifestyle advice can reduce features of fatty liver disease in some children, a significant subset develop worsening disease, particularly with decreasing glycemic control and weight gain. More effective interventions are needed for children who fail to respond to standard lifestyle advice.
Acknowledgments
We gratefully acknowledge the contributions and dedication of the study participants and staff in the NASH CRN.
CRediT Authorship Contributions
Stavra A. Xanthakos, MD (Conceptualization: Supporting; Formal analysis: Equal;
Funding acquisition: Equal; Investigation: Equal; Methodology: Equal; Project
administration: Equal; Supervision: Equal; Writing – original draft: Lead; Writing –
review & editing: Lead). Joel E. Lavine, MD, PhD (Conceptualization: Lead; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Equal; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Equal). Katherine P. Yates, ScM (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Lead; Investigation: Equal; Methodology: Equal; Supervision:
Supporting; Validation: Lead; Writing – original draft: Supporting; Writing – review &
editing: Equal). Jeffrey B. Schwimmer, MD (Conceptualization: Supporting; Formal analysis:
Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting;
Project administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Jean P. Molleston, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Philip Rosenthal, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Karen F. Murray, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Miriam B. Vos, MD, MS (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project
administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Ajay K. Jain, MD, DNB (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Equal). Ann O. Scheimann, MD (Conceptualization:
Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Supporting). Tamir Miloh, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Equal; Writing – review & editing: Supporting). Mark Fishbein, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Supporting; Project
administration: Equal; Supervision: Equal; Writing – review & editing: Supporting). Cynthia A. Behling, MD (Conceptualization: Supporting; Formal analysis: Supporting; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Supervision: Supporting; Writing – review & editing: Equal). Elizabeth M. Brunt, MD (Conceptualization: Supporting; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Equal; Methodology: Equal; Project administration: Equal; Supervision: Supporting; Writing – review & editing: Equal). Arun J. Sanyal, MD (Conceptualization: Supporting; Formal analysis: Supporting;
Funding acquisition: Lead; Investigation: Equal; Methodology: Supporting; Project administration: Supporting; Supervision: Equal; Writing – review & editing: Equal). James Tonascia, PhD (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Funding acquisition: Equal; Investigation: Equal; Methodology: Equal; Project administration: Equal; Supervision: Equal; Writing – review & editing: Supporting).
Funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061713, U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U24DK061730). Additional support is received from the National Center for Advancing Translational Sciences (NCATS) (grants UL1TR000077, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000454). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. The TONIC trial was conducted by the NASH CRN and supported in part by the Intramural Research Program of the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The vitamin E and matching placebo were provided by Pharmavite through a Clinical Trial Agreement with the National Institutes of Health. The CyNCh trial was conducted by the NASH CRN and supported in part by the Intramural Research Program of the National Cancer Institute and by a Collaborative Research and Development Agreement (CRADA) between NIDDK and Raptor Pharmaceuticals.
Abbreviations used in this paper:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- GGT
gamma-glutamyl transferase
- HbA1c
hemoglobin A1c
- LDL
low density lipoprotein cholesterol
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- NASH CRN
Nonalcoholic Steatohepatitis Clinical Research Network
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- OR
odds ratio
- PNPLA3
Patatin-like phospholipase domain-containing protein 3
Appendix 1. NASH CRN Pediatric Clinical Centers and Resource Centers
Baylor College of Medicine, Houston, TX: Stephanie Abrams, MD; Donna Garner, CPNP; Paula Hertel, MD; Ryan Himes, MD; Alicia Lawson, BS; Tamir Miloh, MD; Nicole Triggs, CPNP
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Kristin Bramlage, MD; April Carr, BS, CCRP; Kim Cecil, PhD; Meghan McNeill, MS; Marialena Mouzaki, MD; Andrew Trout, MD; Stavra Xanthakos, MD; Kimberlee Bernstein, BS, CCRP (2012–2018); Stephanie DeVore, MSPH (2009–2011); Rohit Kohli, MD (2009–2016); Kathleen Lake, MSW (2009–2012); Daniel Podberesky, MD (2009–2014); Alex Towbin, MD (2009–2016)
Columbia University, New York, NY: Joel E. Lavine, MD, PhD; Ali Mencin, MD; Elena Reynoso, MD
Emory University, Atlanta, GA: Adina Alazraki, MD; Rebecca Cleeton, MPH, CCRP; Maria Cordero, CCRP; Albert Hernandez; Saul Karpen, MD, PhD; Jessica Cruz Munos (2013–2015); Nicholas Raviele (2012–2014); Miriam Vos, MD, MSPH, FAHA
Indiana University School of Medicine/Riley Hospital for Children, Indianapolis, IN: Molly Bozic, MD; Laura Carr, RN; Oscar W. Cummings, MD; Kathryn Harlow, MD; Ann Klipsch, RN; Jean P. Molleston, MD; Emily Ragozzino; Girish Rao, MD
Johns Hopkins Hospital, Baltimore, MD: Kimberly Kafka, RN; Ann Scheimann, MD
Northwestern University Feinberg School of Medicine/Ann & Robert H. Lurie Children’s
Hospital of Chicago: Mark H. Fishbein, MD; Joy Ito, RN; Saeed Mohammad, MD; Peter F. Whitington, MD (2009–2017)
Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Elizabeth M. Brunt, MD (2002–2008); Danielle Carpenter, MD; Theresa Cattoor, RN; Jose Derdoy, MD (2007–2011); Janet Freebersyser, RN; Ajay Jain MD; Debra King, RN (2004–2015); Jinping Lai, MD (2015–2016); Joan Siegner, RN (2004–2015); Susan Stewart, RN (2004–2015); Susan Torretta; Kristina Wriston, RN (2015)
University of California San Diego, San Diego, CA: Jorge Angeles, MD; Jennifer Arin; Cynthia Behling, MD, PhD; Craig Bross; Carissa Carrier; Jennifer Collins; Diana De La Pena; Janis Durelle; Mary Catherine Huckaby, MD; Joel E. Lavine, MD, PhD (2002–2010); Michael S. Middleton, MD, PhD; Kimberly Newton, MD; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Patricia Ugalde-Nicalo, MD
University of California San Francisco, San Francisco, CA: Jesse Courtier, MD; Ryan Gill, MD, PhD; Camille Langlois, MS; Emily Rothbaum Perito, MD; Philip Rosenthal, MD; Patrika Tsai, MD
University of Washington Medical Center and Seattle Children’s Hospital, Seattle, WA: Niviann Blondet, MD; Kara Cooper; Karen Murray, MD; Randolph Otto, MD; Matthew Yeh, MD, PhD; Melissa Young
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD (2008–2015); Kathryn Fowler, MD (2012–2015)
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Sherry Hall, MS; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (2002–2011); Averell H. Sherker, MD; Rebecca Torrance, RN, MS
Data Coordinating Center, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, MD:
Patricia Belt, BS; Jeanne M. Clark, MD, MPH; John Dodge (2002–2018); Michele Donithan, MHS (2002–2017); Milana Isaacson, BS (2002–2018); Mariana Lazo, MD, PhD, ScM; Jill Meinert; Laura Miriel, BS; Emily P. Sharkey, MPH, MBA; Jacqueline Smith, AA; Michael Smith, BS; Alice Sternberg, ScM; James Tonascia, PhD; Mark L. Van Natta, MHS; Annette Wagoner; Laura A. Wilson, ScM; Goro Yamada, PhD, MHS, MHS, MMS; Katherine P. Yates, ScM
Appendix 2. Inclusion and Exclusion Criteria for TONIC and CyNCH Trials
TONIC Randomized Controlled Trial: enrollment period September 2005 to March 2010
TONIC Inclusion criteria:
1. Children age 8 through 17 years.
2. Histological evidence of NAFLD within 6 months of randomization.
3. ALT level > 60 U/L on 2 separate occasions at least 30 days apart but no more than 12 months apart.
4. Consent.
5. Randomized within 16 weeks of starting screening.
TONIC Inclusion criteria:
1. History of significant alcohol intake or inability to quantify alcohol intake.
- 2. Diabetes mellitus:
- fasting serum glucose ≥ 126 mg/dL;
- or 2 hour serum glucose ≥ 200 mg/dL on oral glucose tolerance test;
- or history of diabetes mellitus determined by site investigator.
3. ALT >400 U/L on measurement closest in time to randomization.
4. Clinical or histological evidence of cirrhosis.
5. Evidence of other causes of chronic liver disease including: alpha-1 antitrypsin deficiency, bile duct anomalies, hemochromatosis, hepatitis (autoimmune or viral), Wilson disease.
6. Serum creatinine ≥ 1.5 mg/dL for male and ≥ 1.4 mg/dL for female individuals.
7. Use of drugs historically associated with hepatic steatosis (systemic glucocorticoids, tetracyclines, anabolic steroids, valproic acid, salicylates, tamoxifen, other known hepatotoxins) for more than 2 weeks in the 2 years before screening.
8. Use of antidiabetic drugs (insulin, biguanides, glucosidase inhibitors, sulfonylureas, meglitinides, metformin, thiazolidinediones) in the 3 months before randomization.
9. Use of anti-NAFLD drugs (metformin, vitamin E, thiazolidinediones, ursodeoxycholic acid, S-adenosylmethionine-e, betaine, milk thistle, probiotics) in the 3 months before randomization.
10. Use of any over-the-counter or herbal remedy for hyperlipidemia in the 3 months before randomization.
11. History of metabolic acidosis.
12. History of renal dysfunction.
13. History of coagulopathy.
14. History of bariatric or hepatobiliary surgery.
15. History of total parenteral nutrition during the past 3 years before screening.
16. Inability to swallow study medication.
17. Vitamin E supplementation > 100 IU per day.
18. Disease considered by study physician to be significant.
19. Females of childbearing potential: positive pregnancy test during screening or randomization or unwillingness to use an effective form of birth control during the trial.
20. Females of childbearing potential: potential breastfeeding.
21. Any other condition which would impeded compliance or hinder completion of study, in the opinion of the investigator.
CyNCH Randomized Clinical Trial: enrollment period June 2012 to January 2014
CyNCH Inclusion criteria:
1. Children age 8 to 17 years.
2. Liver biopsy within 90 days of screening visit and not more than 120 days before randomization.
3. Clinical history consistent with NAFLD.
4. Definite NAFLD based on liver histology.
5. No evidence of any other liver disease by clinical history or histological evaluation.
6. A histological severity of: NAFLD Activity Score (NAS) ≥ 4.
7. Sexually active female participants of childbearing potential must agree to use the same 2 acceptable forms of contraception from screening through completion of the study and to complete a pregnancy test at each study visit.
8. Participants must be able to swallow cysteamine bitartrate delayed release capsules.
9. Written informed consent from parent or legal guardian.
10. Written informed assent from the child.
CyNCH Exclusion Criteria:
1. There will be no exclusion criteria based on race, ethnicity, or gender.
- 2. Participants with a current history of the following conditions or any other health issues that make it unsafe for them to participate in the opinion of the Investigators:
- Inflammatory bowel disease or prior resection of small intestine;
- Heart disease (eg, myocardial infarction, heart failure, unstable arrhythmias);
- Seizure disorder;
- Active coagulopathy;
- Gastrointestinal ulcers/bleeding;
- Renal dysfunction with a creatinine clearance < 90 mL/min/m2;
- History of active malignant disease requiring chemotherapy or radiation within the past 12 months before randomization;
- History of significant alcohol intake or inability to quantify alcohol consumption;
- Chronic use (more than 2 consecutive weeks) of medications known to cause hepatic steatosis or steatohepatitis (systemic glucocorticoids, tetracycline, anabolic steroids, valproic acid, salicylates, tamoxifen) in the past year;
- The use of other known hepatotoxins within 90 days of liver biopsy or within 120 days of randomization;
- Initiation of medications with the intent to treat NAFLD/NASH in the time period following liver biopsy and before randomization;
- History of total parenteral nutrition use in year before screening;
- History of bariatric surgery or planning to undergo bariatric surgery during study duration;
- Clinically significant depression (patients hospitalized for suicidal ideations or suicide attempts within the past 12 months);
- Any female nursing, planning a pregnancy, known or suspected to be pregnant, or who has a positive pregnancy screen.
- 3. Noncompensated liver disease with any one of the following hematologic, biochemical, and serological criteria on entry into protocol:
- Hemoglobin < 10 g/dL;
- White blood cell (WBC) < 3500 cells/mm3 of blood;
- Neutrophil count < 1500 cells/mm3 of blood;
- Platelets < 130,000 cells/mm3 of blood;
- Direct bilirubin > 1.0 mg/dL;
- Total bilirubin >3 mg/dL;
- Albumin < 3.2 g/dL;
- International normalized ratio (INR) > 1.4.
4. Poorly controlled diabetes mellitus (hemoglobin A1c (HbA1c) > 9%).
5. Evidence of other chronic liver disease, including: viral or autoimmune hepatitis, hemochromatosis, alpha-1-antitrypsin deficiency, Wilson disease.
6. Children who are currently enrolled in a clinical trial or who received an investigational study drug within 180 days of screening or liver biopsy.
7. Subjects who are not able or willing to comply with the protocol or have any other condition that would impede compliance or hinder completion of the study, in the opinion of the investigator.
8. Failure to give informed consent.
Appendix 3. Synopsis of standard of care lifestyle counseling provided to all participants.
Both trials provided written dietary and lifestyle advice to participants at each study visit and recommendations were discussed with participants by trained study staff. These guidelines covered similar information in both studies and are aligned with American Academy of Pediatrics’ 2007 Expert Committee Recommendations Regarding Prevention, Assessment and Treatment of Child and Adolescent Overweight and Obesity.15 Specific recommendations provided to participants and their families included:
Offer healthy choices at home and do not keep tempting unhealthy foods around the home.
Aim for at least 5 servings of fruit and vegetables per day.
Offer appropriate portion sizes.
Limit intake of added sugar intake to no more than 1 serving on 1 to 2 days per week. Change from sugar-sweetened beverages to water or noncaloric beverages.
Consume less than 30% of calories from fat (avoid fried foods, select non- or low-fat dairy products, use unsaturated oils for cooking).
Avoid foods containing saturated and trans fats. Goal less than 10% of calories from saturated fats.
Have regular family mealtimes, offering 3 portion-controlled healthy meals and 1–2 portion-controlled healthy snacks per day.
Eat at table with television off.
Limit fast food and make healthier choices when eating out.
Encourage 1 hour of moderate to vigorous physical activity each day, 5 times per week, defined as activity that is sufficient to break a sweat or raise heart rate to 130 beats per minute.
Limit TV viewing and screen time to <2 hours per day.
Model healthy eating and screen time behavior in the family.
If overweight or obese, advised to aim for a modest total caloric restriction, calculated from expected daily caloric needs based on height and age, by trained clinic staff. Very overweight participants (BMI ≥ 95th percentiles for age and gender) were given target goal of losing weight at rate of 2–4 pounds per month down to BMI < 85th percentile, per expert committee recommendations.
Footnotes
Conflict of Interest
These authors disclose the following: Cynthia Behling is a consultant to ICON and Covance. Elizabeth Brunt is a consultant to Perspectum Diagnostics and Histoindex. Ajay Jain is a consultant to Alexion. Joel Lavine is a consultant for Surrozen, Intercept, and Novo Nordisk. He received grant support from Genfit. Tamir Miloh is a consultant to Alexion. Jean Molleston has received research support from Gilead, Abbvie, Albireo, and Shire/Mirum. Karen Murray is a consultant to Gilead and Albireo. Philip Rosenthal has received research support from Gilead, Abbvie, BMS, Roche/Genentech, Retrophin, Merck, Albireo, Mirum, and Arrowhead and has served as a consultant for Albireo, Audentes, Retrophin, Gilead, Abbvie, Dicerna, Arrowhead and Mirum. Arun Sanyal is president of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect Inversago, and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Bristol Myers Squibb, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate. Ann Scheimann has received research support from Millendo Therapeutics, Insys Therapeutics, Albireo, and serves on a Data Safety Monitoring Board for Levo Therapeutics. Jeffrey Schwimmer has received grants from Galmed, Genfit, and Intercept. Miriam Vos has received grants from Gemphire, Resonance Health, and Shire, and is a consultant for Boehringer Ingelheim, Bristol Myers Squibb, Immuron, Intercept, Mallinckrodt, Novo Nordisk, Target Pharmasolutions, and AMRA. Stavra Xanthakos has received grants from Axcella Health and Target PharmaSolutions. She receives royalties from UptoDate. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.07.034.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 3.Wattacheril J, Lavine JE, Chalasani NP, et al. Genome-wide associations related to hepatic histology in nonalcoholic fatty liver disease in Hispanic boys. J Pediatr 2017;190:100–107.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 2014;147:754–764. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki A, Abdelmalek MF, Schwimmer JB, et al. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2012; 10:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr 2016;170: e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton HM, Yates K, Unalp-Arida A, et al. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol 2010;105:2093–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of nonalcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 2009;58:1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molleston JP, White F, Teckman J, et al. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol 2002;97:2460–2462. [DOI] [PubMed] [Google Scholar]
- 11.Kohli R, Boyd T, Lake K, et al. Rapid progression of NASH in childhood. J Pediatr Gastroenterol Nutr 2010; 50:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HH AK, Henderson J, Vanhoesen K, et al. Nonalcoholic fatty liver disease in children: a single center experience. Clin Gastroenterol Hepatol 2008;6:799–802. [DOI] [PubMed] [Google Scholar]
- 13.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Lavine JE, Wilson LA, et al. In children with nonalcoholic fatty liver disease, cysteamine bitartrate delayed release improves liver enzymes but does not reduce disease activity scores. Gastroenterology 2016;151:1141–1154.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120(Suppl 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 17.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011;53:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkhouri N, Hanouneh IA, Zein NN, et al. Liver transplantation for nonalcoholic steatohepatitis in young patients. Transpl Int 2016;29:418–424. [DOI] [PubMed] [Google Scholar]
- 19.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–654; e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argo CK, Northup PG, Al-Osaimi AM, et al. Systematic review of risk factors for fibrosis progression in nonalcoholic steatohepatitis. J Hepatol 2009;51:371–379. [DOI] [PubMed] [Google Scholar]
- 22.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 23.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008;49:608–612. [DOI] [PubMed] [Google Scholar]
- 24.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009;7:234–238. [DOI] [PubMed] [Google Scholar]
- 25.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43:617–649. [DOI] [PubMed] [Google Scholar]
- 26.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 27.Cholankeril G, Wong RJ, Hu M, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci 2017; 62:2915–2922. [DOI] [PubMed] [Google Scholar]
- 28.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;376:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009;49:809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;317:2427–2444. [DOI] [PubMed] [Google Scholar]
- 31.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128:1689–1712. [DOI] [PubMed] [Google Scholar]
- 32.Inge TH, Xanthakos SA. Reversal of nonalcoholic steatohepatitis in adolescents after metabolic surgery. J Pediatr 2017;180:6–7. [DOI] [PubMed] [Google Scholar]
- 33.Vuppalanchi R, Unalp A, Van Natta ML, et al. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson JA, Konomi JV, Mendoza MV, et al. Performance of fibrosis prediction scores in paediatric nonalcoholic fatty liver disease. J Paediatr Child Health 2018;54:172–176. [DOI] [PubMed] [Google Scholar]
- 35.Schwimmer JB, Middleton MS, Behling C, et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015; 61:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwimmer JB, Behling C, Angeles JE, et al. Magnetic resonance elastography measured shear stiffness as a biomarker of fibrosis in pediatric nonalcoholic fatty liver disease. Hepatology 2017;66:1474–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouzaki M, Trout AT, Arce-Clachar AC, et al. Assessment of nonalcoholic fatty liver disease progression in children using magnetic resonance imaging. J Pediatr 2018;201:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vos MB, Dimick-Santos L, Mehta R, et al. Factors to consider in the development of drugs for pediatric nonalcoholic fatty liver disease. Gastroenterology 2019; 157:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.