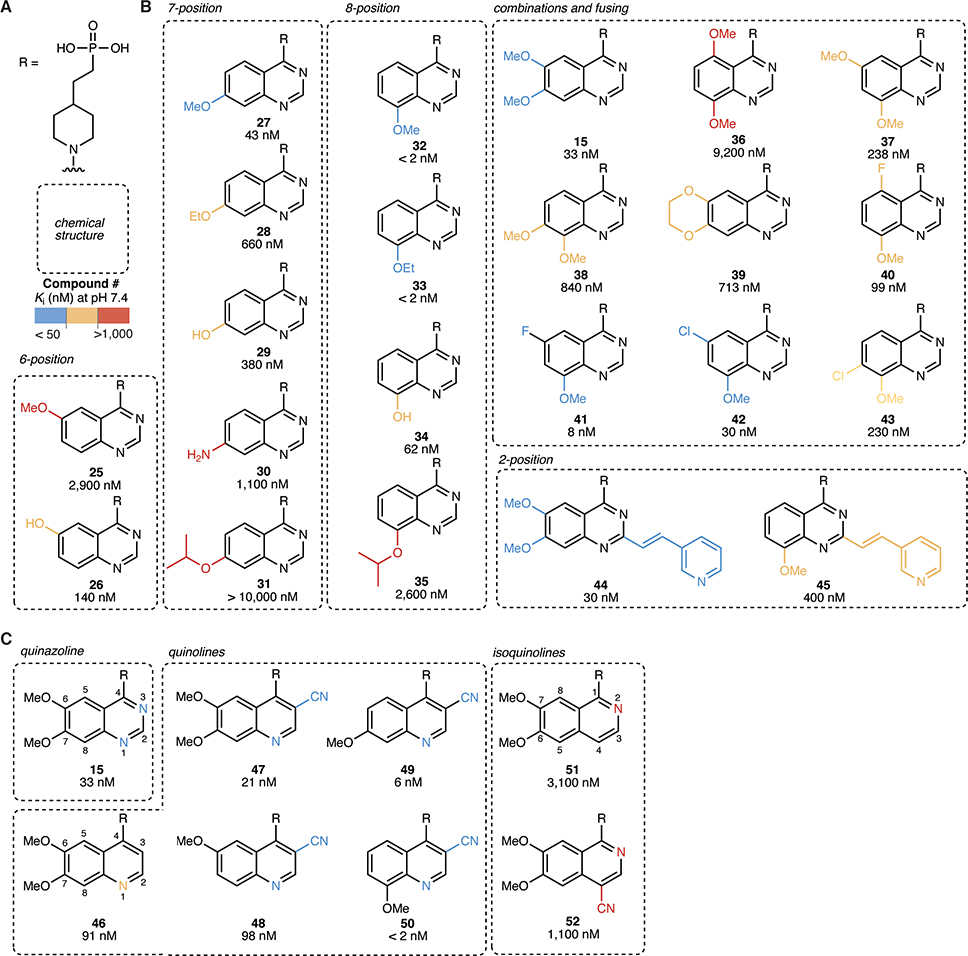
Figure 5 |. 8-methoxy quinazoline and 3-nitrile quinoline tails achieve high potency.
(A) Chemical structures of the R group (head = phosphonate, core = piperidine). (B)–(C) Chemical structures of quinazoline (B) and quinoline (C) tails with corresponding Ki values (mean of at least 2 independent replicates) at pH 7.4. Ki values were determined using 3 nM ENPP1 and 5 μM cGAMP.