Fig. 6. Mechanism of ER tubulation by D-octadecapeptide.
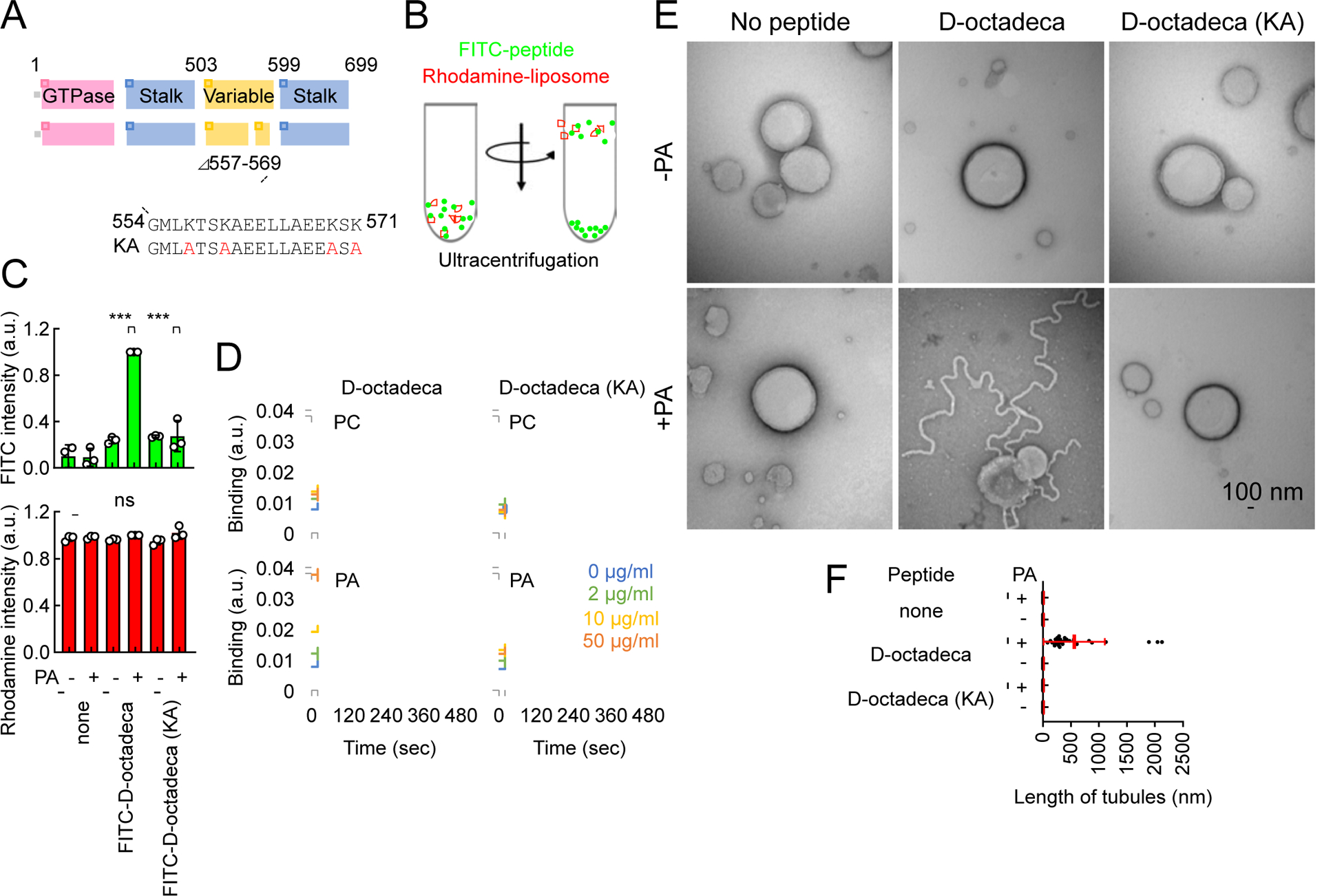
(A) The amino acid sequence of D-octadecapeptide is shown. The KA D-octadecapeptide mutant was created by changing four lysines to alanines (red). (B) Liposome flotation assay. Synthetic liposomes that mimic the ER membrane (which contains PC, PE, PI, and PS) carrying rhodamine-PE with or without saturated PA were incubated with FITC-labeled D-octadecapeptide or KA D-octadecapeptide and placed at the bottom of a sucrose gradient. (C) After ultracentrifugation, we collected four fractions from the top of the tube and measured FITC and rhodamine fluorescence. Almost 100% of the liposomes floated to the top fraction based on rhodamine fluorescence. Bars are average ± SD (n = 3 experiments). (D) Interactions of D-octadecapeptide and KA D-octadecapeptide with saturated PC or saturated PA were measured using surface plasmon resonance. Averages of triplicates for each lipid concentration are shown. (E) The same liposomes used in (B) were incubated with 1 μM D-octadecapeptide or KA D-octadecapeptide and viewed with negative-stain EM. (F) The length of the tubules that emerged from the liposomes was quantified. Bars are average ± SD (n = 20 tubules). Statistical analysis was performed using one-way ANOVA with the Tukey post-hoc test (C) and Kruskal-Wallis with Dunn’s post-hoc test (F): ***p<0.001. See also Figure S4.
