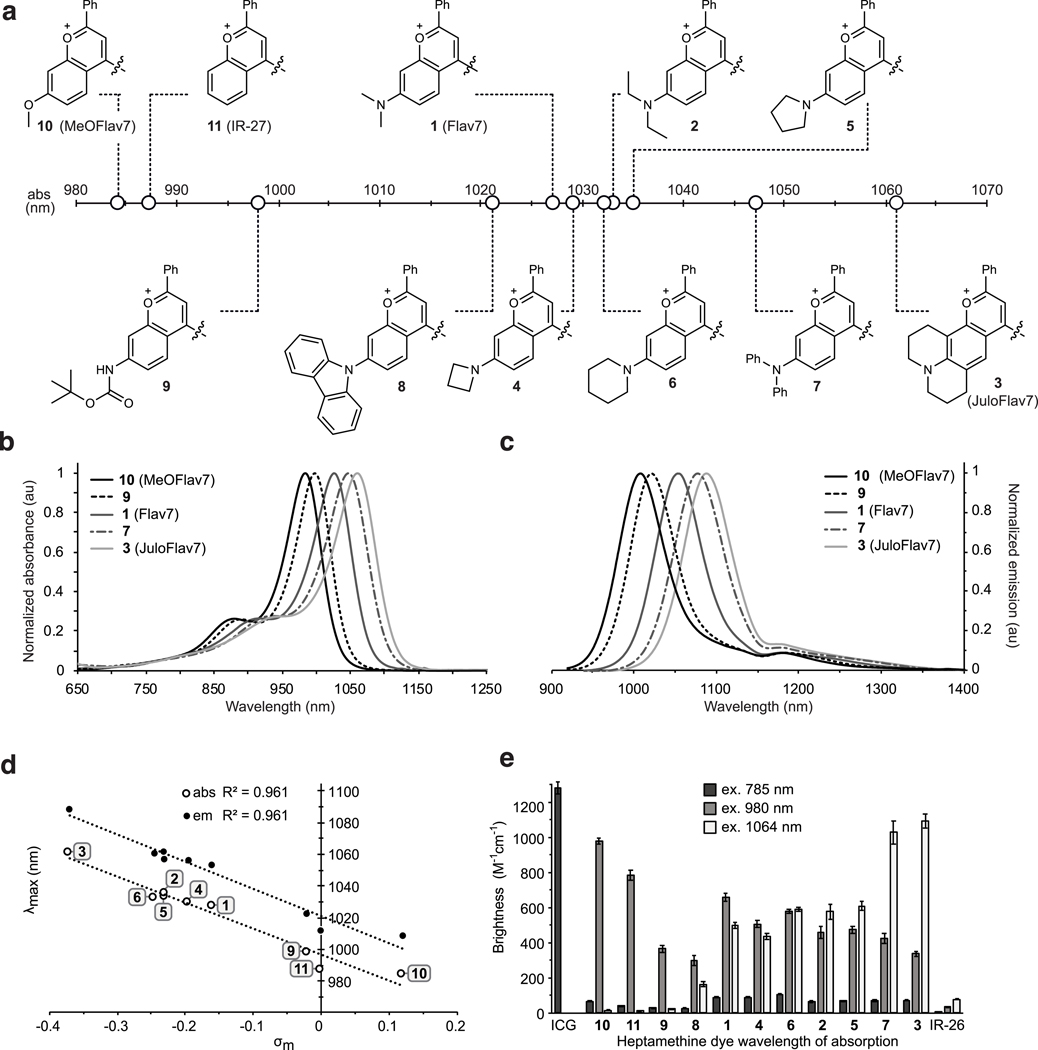
Fig. 2.
Panel of flavylium heptamethine dyes and their photophysical properties. a) Heterocycle structures and absorption wavelength maxima visualized graphically on the electromagnetic spectrum. b) Absorption and c) Emission profiles (ex. 885 nm) of selected polymethine dyes. d) Hammett plot relating substituent constants (ref. 50) to absorption and emission wavelengths of dyes 1–6 and 9–11. e) Brightness (defined as ) of the heptamethine derivatives at relevant excitation wavelengths ( = 785 nm (dark grey), = 980 nm (light grey), and = 1064 nm (white). Error bars represent the propagated error from standard deviations in and measurements.