Abstract
The importance of oxygen to life has been recognized for hundreds of years, but how cells and tissues sense reduced oxygen levels remained elusive until the late twentieth century. The 2019 Nobel Prize in Physiology or Medicine was awarded to William G. Kaelin Jr., Sir Peter J. Ratcliffe, and Gregg L. Semenza for their discovery of hypoxia-inducible factor, a key transcription factor that regulates gene expression in response to decreases in cellular oxygenation. The three scientists provided the first information about the cellular oxygen-sensing mechanism and downstream signal transduction under hypoxic conditions. Their discoveries have also paved the way for promising novel treatments for cancer, renal anemia, and inflammatory disease.
Keywords: Hypoxia-inducible factor, von hippel-Lindau (VHL) disease, Nobel Prize
The three pioneers
Oxygen is one of the most important molecules that enable life on Earth. Mammals have evolved sophisticated regulatory mechanisms to ensure that every organ receives sufficient oxygen to sustain normal physiological functions. One well-known example is hypoxia-induced tachypnea, a natural reflex critical for maintaining homeostasis during changes in arterial blood oxygen saturation [1]. However, acclimatization of the human body to decreased oxygen availability relies not only on the rise in ventilation and redistribution of blood flow to vital organs, but also on stimulation of hemoglobin production. The latter physiological response, i.e. upregulation of erythropoietin (EPO) expression to increase red blood cell synthesis, has been known since the early 1970s [2]. However, the precise underlying mechanisms remained poorly understood until the late twentieth century when Gregg Semenza, Peter Ratcliffe, and William Kaelin made a series of breakthroughs that opened the door to new avenues of hypoxia research.
In 1991, Gregg Semenza identified a DNA sequence in the 3′-flanking region of the human EPO gene that functioned as a hypoxia-inducible enhancer [3]. This same finding was confirmed immediately by Peter Ratcliffe's team using mouse EPO gene [4]. Using electrophoretic mobility shift assays, Semenza discovered a nuclear protein complex, designated hypoxia-inducible factor (HIF), that was detected in hypoxic, but not in normoxic cells [5]. However, these findings were unlikely to attract considerable attention if HIF induction was simply a response to EPO upregulation during hypoxia. A year later, Semenza and Ratcliffe published the same finding separately almost at the same time that HIF signaling also operated in non-EPO producing hypoxic cells, implying the existence of a ubiquitous oxygen-sensing mechanism in mammalian cells [6,7]. This finding also suggested that HIF could target genes other than EPO. In 1995, Semenza's team identified the genes encoding HIF and purified the protein, which was composed of HIF-1α and HIF-1β subunits [8,9]. This work was then used to generate antibodies that allowed researchers to characterize cellular expression of HIF-1α as a function of oxygen level.
Research into the nature of the oxygen sensor that regulates HIF activity continued into the late 1990s. Several groups showed that under hypoxic conditions, HIF-1α protein levels soared without corresponding increase in mRNA levels, suggesting regulation at the level of protein synthesis or degradation [10,11]. It became clear that in oxygenated cells, HIF-1α protein degradation is mediated by a distinct oxygen-dependent degradation domain (ODD) via the proteasome pathway [12,13]. Meanwhile, William Kaelin was studying von Hippel-Lindau (VHL) disease, which is caused by germline mutations in VHL tumor suppressor gene [14]. VHL-associated neoplasms, such as renal cell carcinoma and hemangioblastoma, are remarkable for high levels of vascular endothelial growth factor (VEGF) and tumor vascularity. Kaelin suggested that VHL could be involved in how cancer cells stabilize hypoxia-inducible mRNA, such as VEGF mRNA [15,16], but how the interaction between VHL and HIF-1α was regulated by oxygen remained unclear. The actual breakthrough was made by the Ratcliffe lab when they showed that, in the presence of oxygen and iron, HIF-1α is targeted for proteasomal destruction by an E3 ubiquitin ligase complex containing the VHL protein (pVHL) [17]. This observation was confirmed by the Kaelin lab [18] and other groups [19]. In 2001, teams led by Kaelin and Ratcliffe separately provided the final piece to the puzzle when they discovered that oxygen-regulated prolyl hydroxylation of HIF-1α mediates binding of pVHL to the ODD of HIF-1α [20,21]. Ratcliffe reported on three different prolyl hydroxylase domains (PHD) and demonstrated that their ability to modify HIF-1α requires molecular oxygen as a co-substrate. This finding provided long-sought insight into how cellular oxygen regulates HIF-1α expression [22].
Oxygen-dependent regulation of HIF
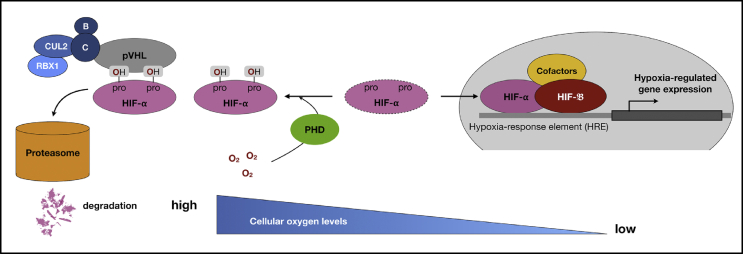
Thanks to the pioneering works of Gregg Semenza, Peter Ratcliffe, and William Kaelin, we now know that when intracellular oxygen levels reach a critical threshold, HIF-1α is modified by PHD, which targets it for degradation (Fig. 1). Hydroxylation of specific proline residues in the ODD region of HIF-1α increases the affinity of HIF-1α for pVHL by at least three orders of magnitude [23]. Conversely, when cells are exposed to hypoxia, prolyl hydroxylation is suppressed and HIF-1α translocates to the nucleus where it forms a functional complex with HIF-1β and other co-activators, such as cofactor p300. The complex binds to hypoxia response elements in the promoter/enhancer region of genes, which are associated with a broad range of transcriptional targets. Subsequent studies showed that these target genes are not only involved in both systemic responses to hypoxia, angiogenesis and erythropoiesis, but also in cellular responses, such as alteration of glucose metabolism. HIF-mediated hypoxia response is increasingly recognized as an important determinant of disease outcomes including cancer and inflammatory disease [[24], [25], [26], [27]].
Fig. 1.
Cellular regulation of hypoxia-inducible factor (HIF) activity. Intracellular oxygen level determines the accessibility of HIF-1α being hydroxylized by PHDs. In normoxia, prolyl hydroxylation of HIF-1α increases the affinity of HIF-1α for pVHL which recruits elongin B and C, CULs, and RBX1 to constitute a functional E3 ubiquitin ligase, thereby leading HIF-1α into proteasomal degradation. When oxygen becomes limited, HIF-1α is no longer hydroxylated and becomes stabilized. HIF-1α then binds to HIF-1β and the HIF-1 heterodimer binds to HRE that ultimately results in the transcriptional responses to hypoxia. Abbreviations used: HIF: hypoxia-inducible factor; PHD: prolyl hydroxylase domain; pVHL: Von Hippel–Lindau protein; CUL2: Culin 2; B: Elongin B; C: Elongin C; RBX1: RING-box protein 1; HRE: hypoxia-response element.
Perspectives
Hypoxia is a known inducer of angiogenesis through HIF-dependent induction of VEGF expression [28]. Furthermore, metabolic adaptations of cancer cells by HIF-1 in response to hypoxia, e.g., the switch from oxidative phosphorylation to glycolysis, using glutamine rather than glucose for lipid synthesis, and drop in extracellular pH, are known to be involved in driving tumor progression and metastasis [24,29]. Increased HIF-1α levels are associated with increased mortality in many human cancers [24]. Targeting HIF and relevant metabolic enzymes may impair the metabolic flexibility of cancer cells and sensitize them to anticancer drugs [29,30]. Indeed, clinical studies of HIF inhibitors in patients with advanced cancers report encouraging results, and several phase II trials are ongoing [31].
HIF-1 also plays a critical role in the immune response [[32], [33], [34]]. Its induction is essential for infiltration and activation of myeloid cells and HIF-1α knockout myeloid cells show decreased bactericidal capacity [32]. Additionally, HIF-1α regulates the balance between regulatory T cells (Tregs) and Th17 cells. Mice with T cell-specific HIF-1α knockout are resistant to induction of autoimmune encephalitis due to impairment of Th17 responses [33]. Interestingly, similar to cancer cells adapting their metabolism to low oxygen levels, HIF-1α-dependent metabolic switch to glycolysis promotes production of inflammatory Th17 cells while suppressing Treg generation [34], suggesting that HIF-1α inhibitors could ameliorate Th17-mediated inflammation in autoimmune encephalitis. These concepts opened a new field of study called immunometabolism and accumulating data support the view that understanding how metabolism regulates immune cell function could provide new therapeutic opportunities for the many diseases associated with immune system dysregulation. Inflammation can induce hypoxia as a result of increased metabolism and diminished oxygen delivery to inflamed areas. HIF-induced transcriptional changes profoundly impact outcomes of various inflammatory and ischemic conditions, such as inflammatory bowel disease, acute kidney injury, and ischemic-reperfusion injury, and pharmacological modulation of HIF activity may represent a feasible new strategy to their treatment [35]. One of the most exciting possibilities is the use of PHD inhibitors to manage renal anemia. Roxadustat was recently shown to markedly increase endogenous EPO production and upregulate hemoglobin levels in a randomized trial of patients with chronic kidney disease [36]. All of these pivotal achievements, which greatly benefit our patients, build on the earlier works of the three pioneers. The 2019 Nobel Prize in Physiology or Medicine was awarded to honor their discovery of molecular mechanisms that mediate cellular oxygen sensing.
Funding source
The authors declare that they have no conflicts of interest and acknowledge the financial support of grants from Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan (CMRPG3F1183).
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Gonzalez C., Almaraz L., Obeso A., Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 2.Abbrecht P.H., Littell J.K. Plasma erythropoietin in men and mice during acclimatization to different altitudes. J Appl Physiol. 1972;32:54–58. doi: 10.1152/jappl.1972.32.1.54. [DOI] [PubMed] [Google Scholar]
- 3.Semenza G.L., Nejfelt M.K., Chi S.M., Antonarakis S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugh C.W., Tan C.C., Jones R.W., Ratcliffe P.J. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3' to the mouse erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:10553–10557. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G.L., Semenza G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G.L., Jiang B.H., Rue E.A., Semenza G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 10.Gradin K., McGuire J., Wenger R.H., Kvietikova I., fhitelaw M.L., Toftgard R. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L.E., Arany Z., Livingston D.M., Bunn H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 12.Salceda S., Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 13.Huang L.E., Gu J., Schau M., Bunn H.F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliopoulos O., Kibel A., Gray S., Kaelin W.G., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 15.Iliopoulos O., Levy A.P., Jiang C., Kaelin W.G., Jr., Goldberg M.A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaelin W.G., Jr., Maher E.R. The VHL tumour-suppressor gene paradigm. Trends Genet. 1998;14:423–426. doi: 10.1016/s0168-9525(98)01558-3. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 18.Ohh M., Park C.W., Ivan M., Hoffman M.A., Kim T.Y., Huang L.E. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto K., Makino Y., Pereira T., Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 21.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 22.Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O'Rourke J., Mole D.R. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 23.Hon W.C., Wilson M.I., Harlos K., Claridge T.D., Schofield C.J., Pugh C.W. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417:975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 24.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummins E.P., Berra E., Comerford K.M., Ginouves A., Fitzgerald K.T., Seeballuck F. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103:18154–18159. doi: 10.1073/pnas.0602235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummins E.P., Seeballuck F., Keely S.J., Mangan N.E., Callanan J.J., Fallon P.G. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H., Gilbert V., Liu Q., Kapitsinou P.P., Unger T.L., Rha J. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol. 2012;188:5106–5115. doi: 10.4049/jimmunol.1103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wigerup C., Pahlman S., Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Fallah J., Rini B.I. HIF inhibitors: status of current clinical development. Curr Oncol Rep. 2019;21:6. doi: 10.1007/s11912-019-0752-z. [DOI] [PubMed] [Google Scholar]
- 32.Cramer T., Yamanishi Y., Clausen B.E., Forster I., Pawlinski R., Mackman N. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig H.K., Bratton D.L., Colgan S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N., Hao C., Peng X., Lin H., Yin A., Hao L. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]