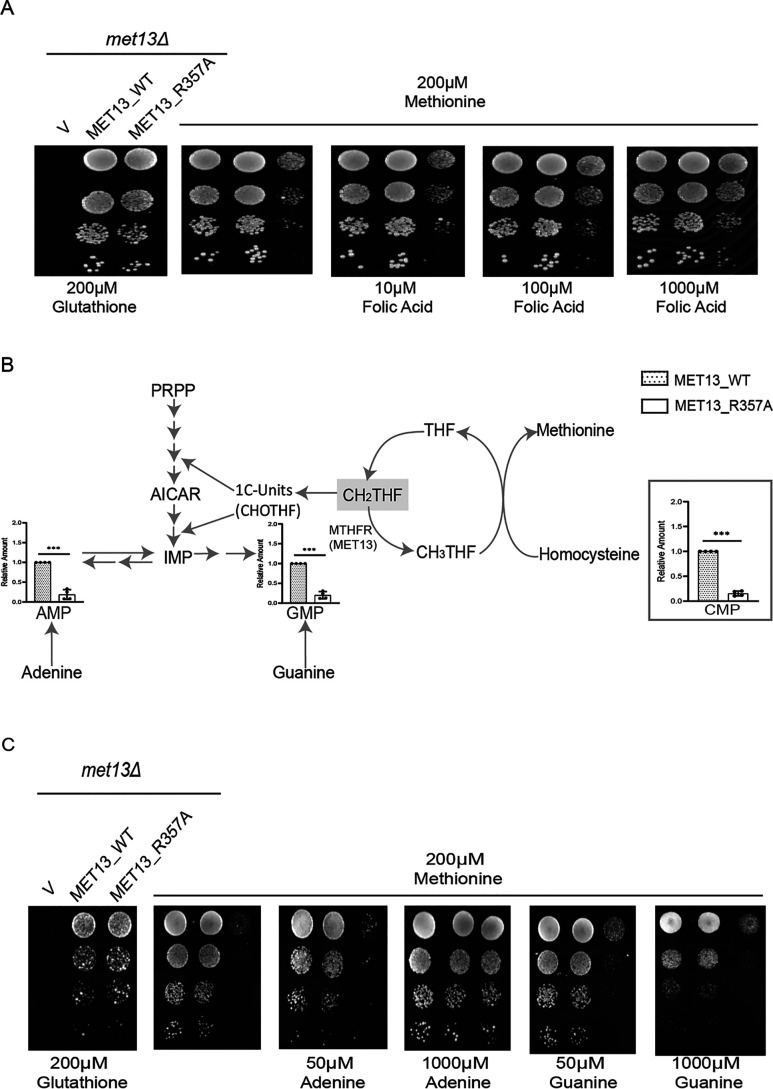
Figure 4.
MTHFR deregulation disrupts the cellular folate and nucleotide pools. A, yeast met13Δ strains transformed with vector control (V), MET13_WT, and MET13_R357A were grown to the exponential phase in minimal medium containing 200 μm GSH, harvested, washed, resuspended in water, and serially diluted to give 0.1, 0.01, 0.001, and 0.0001 A600 of cells. 10 μl of these dilutions were spotted on minimal medium containing either 200 μm GSH, methionine, and methionine along with the increasing concentrations of folic acid (10–1000 μm). The photographs were taken after 72 h of incubation at 30 °C. The experiment was repeated three times, and a representative data set is shown. B, the relative abundance of nucleotide pools in transformants bearing the WT MET13 or deregulated mutant of yeast MTHFR, MET13_R357A. Transformants were grown overnight in SD minimal medium along with GSH (200 μm) as a sulfur source and reinoculated to medium containing methionine (200 μm), and different nucleotide intermediates were determined by LC–MS/MS. The graphs show a representative data set of three biological replicates. C, S. cerevisiae met13Δ strains transformed with vector control (V), and MET13_WT and MET13_R357A were grown to exponential phase in minimal medium containing 200 μm GSH, harvested, washed, resuspended in water, and serially diluted to give 0.1, 0.01, 0.001, and 0.0001 A600 of cells. 10 μl of these dilutions were spotted on minimal medium containing 200 μm GSH, 200 μm methionine, and different concentrations of either adenine or guanine (50–1000 μm) in methionine-supplemented plates. The photographs were taken after 48 h of incubation at 30 °C. The experiment was repeated three times, and a representative data set is shown.