Figure 1.
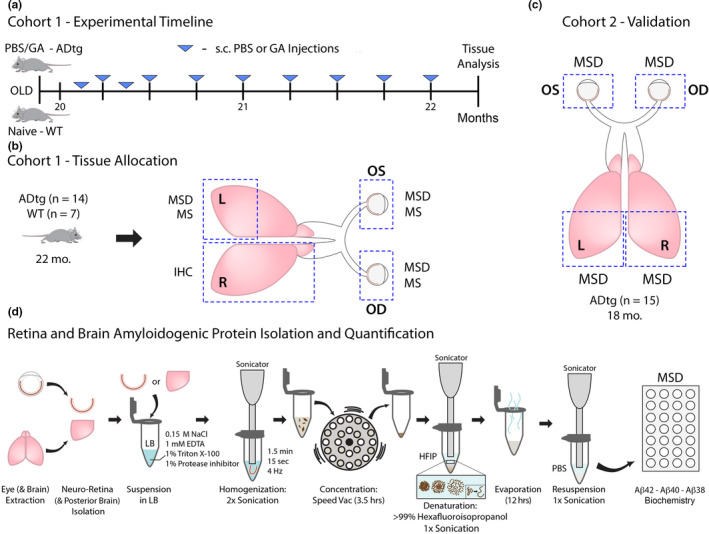
Experimental design and intervention timeline assessing cerebral and retinal tissues in old, late‐stage ADtg mice. (a) Experimental timeline for mouse Cohort 1: 20‐month‐old APPSWE/PS1ΔE9 (ADtg) mice underwent weekly, subcutaneous injections of glatiramer acetate (GA, also known as Copaxone®; 100 µg) for a 2‐month duration (n = 7 mice). Age‐ and sex‐matched ADtg mice were subcutaneously injected with PBS in the same regimen and naïve non‐transgenic (WT) littermates were used as controls (n = 7 mice per group). (b) One week following the last injection, mice were sacrificed, and tissues were collected as described. Paired brains and eyeballs from Cohort 1 were allocated for further analyses: the right (R) cerebral hemisphere was used for immunohistochemistry (IHC), the left (L) posterior brain was used for quantitative biochemical Meso Scale Discovery (MSD) and Mass Spectrometry (MS) assays, and both oculus sinister (OS, left) and oculus dexter (OD, right) eyeballs were collected, the neurosensory retinae isolated, and proteins assessed by MSD and MS analyses. OS and OD retinae were separately analyzed by MSD and pooled together for MS analysis. (c) Cohort 2 was comprised of old ADtg mice (n = 15 mice; average age of 18 months). L and R posterior brains as well as OS and OD eyeballs were collected and analyzed separately for Aβ proteins by MSD. (d) Preparation of mouse neuro‐retina and posterior brain Aβ proteins for quantification by MSD. Each tissue was prepared separately for analysis (OS retina, OD retina, and left and right posterior brains). The protocol involves suspension in lysis buffer (LB), homogenization via sonication, concentration with speed vac, and protein denaturation with hexafluoroisopropanol (HFIP) followed by evaporation and resuspension in PBS prior to protein concentration analysis
