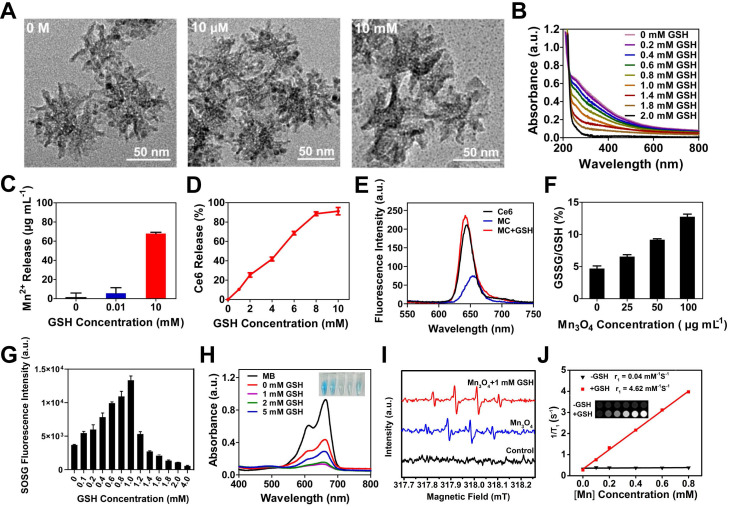
Figure 2.
GSH-activatable generation of 1O2 and ·OH. (A) TEM images of MC@DMSN treated with 0 M, 10 μM and 10 mM GSH for 30 min. (B) UV-vis absorption spectra of Mn3O4 treated with different concentrations of GSH. (C) The Mn2+ release of MC after treated with different concentrations of GSH. (D) GSH-dependent release profiles of Ce6 from MC as measured by the fluorescence spectrophotometer. (E) Fluorescence intensity of Ce6, MC and MC treated with 1 mM GSH. (F) The transformation of GSH to GSSG after treated with different concentrations of Mn3O4. (G) Response of 1O2 generated by MC to different concentrations of GSH. (H) UV-vis absorption spectra and photo (inset) of MB after degraded by Mn3O4 NPs treated with different concentrations of GSH. The concentration of H2O2 is 8 mM. (I) ESR spectra of different reaction systems with DMPO as the spin trap. (J) T1 relaxivity of SRG pretreated with 0.5 mg ml-1 HAase in the absence and presence of 10 mM GSH. The [Mn] concentration was 0, 0.1, 0.2, 0.4, 0.6 and 0.8 mM. The insets were corresponding T1-weighted images.