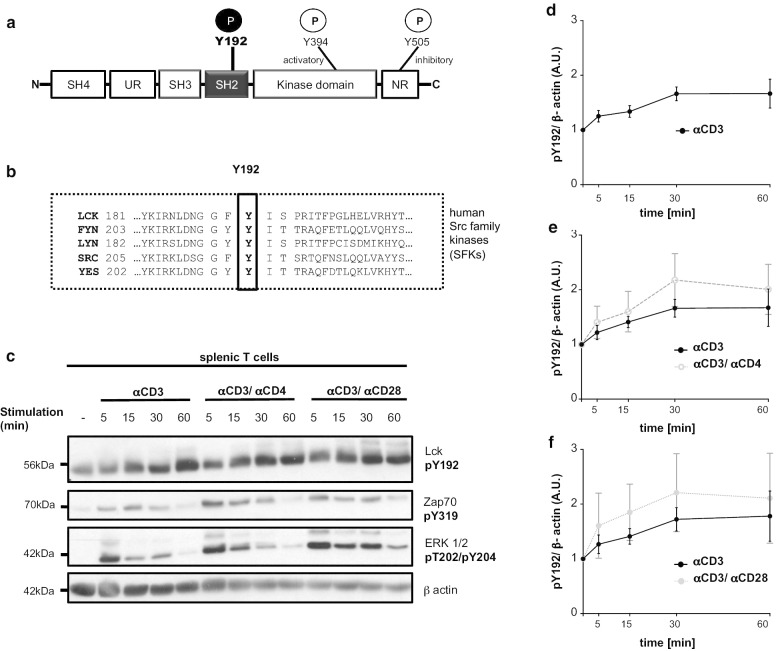
Fig. 1.
LckY192 is inducibly phosphorylated upon TCR stimulation in splenic murine T cells. a Cartoon showing the structure of Lck including the regulatory domains and tyrosine phosphorylation sites. b Lck-Y192 is conserved among Src-family kinases. The amino acid sequences of representative human Src-family kinases are shown. c Murine splenic T cells were stimulated with a CD3 antibody immobilized on microbeads for the indicated periods of time. Co-stimulation occurred with either CD4 or CD28 antibodies. Subsequently, cells were lysed and immunoblot analyses were carried out using a pY192-phosphospecific antibody to monitor phosphorylation dynamics of Y192. The efficiency of T-cell activation was measured using phospho-Zap70 and phospho-Erk1/2 antibodies, respectively. Equal protein loading was verified using a β-actin antibody. PhosphoY192 signals from splenic T cells stimulated with CD3 alone (n = 5) (d), CD3/CD4 (n = 4) (e), or CD3/CD28 (n = 3) (f) were normalized to β-actin and quantified. For the densiometric analysis the median of the Western blot bands were taken. Mean values ± SEM of the indicated experiments are shown