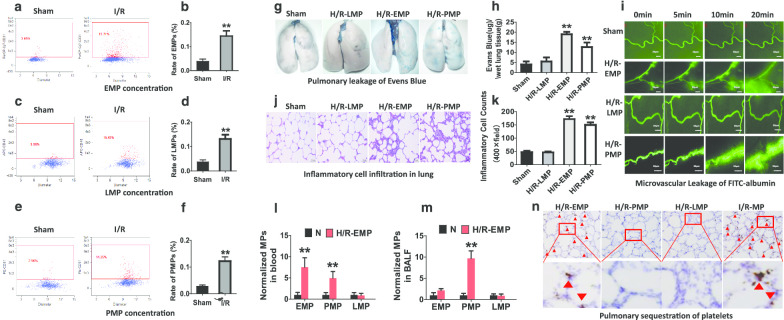
Fig. 2.
The changes of EMPs, PMPs and LMPs in blood after I/R and their role in pulmonary vascular hyperpermeability and lung injury. a, b The change of EMPs in blood marked with CD31 antibodies. c, d The change of LMPs in blood marked with CD45 antibodies. e, f The change of PMPs in blood marked with CD61 antibodies. g, h Effects of H/R-EMPs, H/R-PMPs and H/R-LMPs on pulmonary vascular permeability reflected by Evans Blue leakage. i The effect of H/R-EMPs, H/R-PMPs and H/R-LMPs on leakage of FITC (fluorescein isothiocyanate)-labeled albumin in microvasculature. j, k The status of inflammatory cells infiltration in lung 2 h after intravenous administration of H/R-EMPs, H/R-PMPs and H/R-LMPs. The numbers of inflammatory cells were determined in 10 randomly selected nonoverlapping fields at 400 × magnification in respective sections of the individual rat lung. l, m The effects of H/R-EMPs on the concentrations of EMPs, PMPs and LMPs in plasma (l) and BALF (bronchoalveolar lavage fluid) (m). n The effects of H/R-EMPs, H/R-PMPs and H/R-LMPs on pulmonary sequestration of platelets by immunohistochemical staining, CD41 antibodies were used to mark platelets. The data are the mean ± SD of n experiments (n = 8). NC-LMP, NC-EMP, NC-PMP: derived from normal leukocytes, vascular endothelial cells and platelets, respectively; H/R-LMP, H/R-EMP, H/R-PMP: derived from hypoxia/reoxygenation-treated leukocytes, vascular endothelial cells and platelets, respectively. **P < 0.01 versus N or sham group