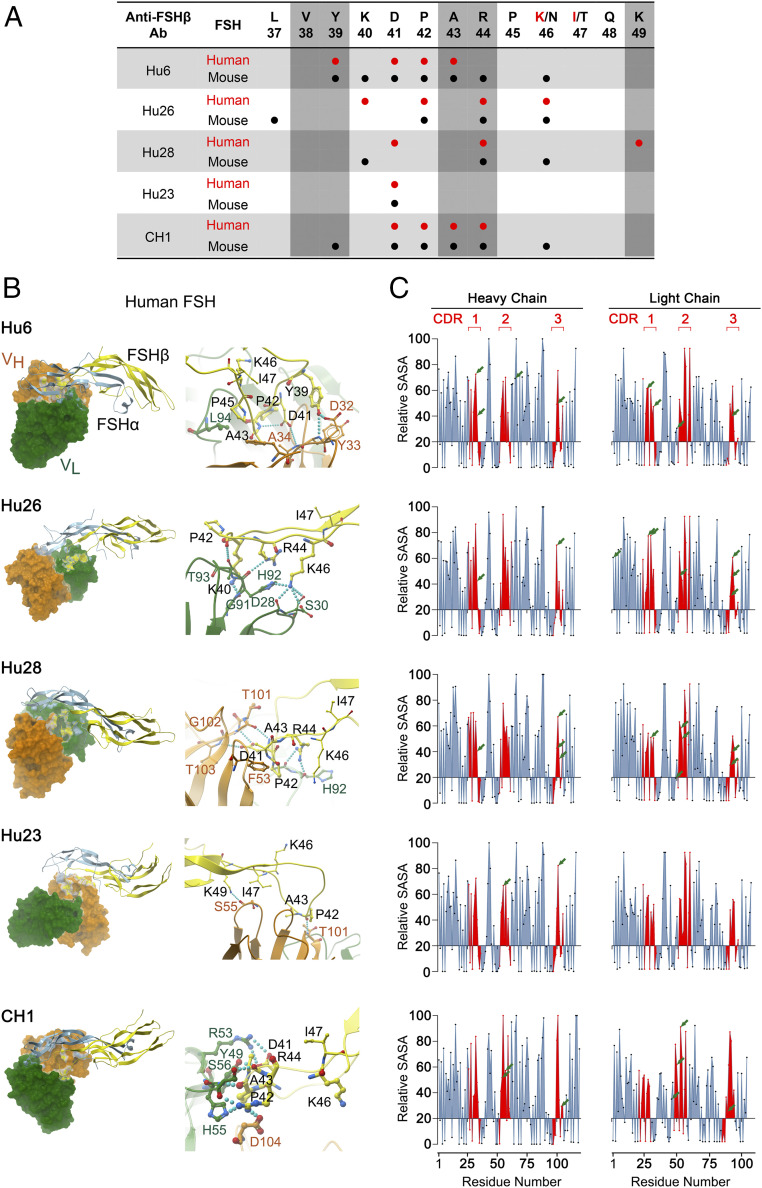
Fig. 2.
Atom-level fine mapping of antibody–FSH interfaces and solvent accessibility of Interacting residues. (A) In silico fine mapping of residues of the targeted 13-amino-acid–long human or mouse FSHβ epitope that interact with specific residues of the variable chain of the antibodies Hu6, Hu26, Hu28, and Hu23, as well as the mouse–human chimera (CH1). The five residues that are known to interact with the FSHR (28) have been identified by vertical shades. (B) Shown are computational models and fine maps of complexes between the α (blue) and β (yellow) subunits of human FSH and the variable regions, VL (green) and VH (orange), of the antibodies. Interactions between specific amino acids are shown (SI Appendix, Fig. S4). (C) Calculated relative SASA values [derived from GETAREA (57, 58); Methods] of individual amino acids within VH and VL regions, with CDRs shown in red. Values below 20 represent an “in” configuration on GETAREA, which reflects solvent-inaccessible (buried) residues. Residues that interact with human FSH are shown by arrows, with ∼70% having SASAs ≥50, indicating good solvent accessibility.