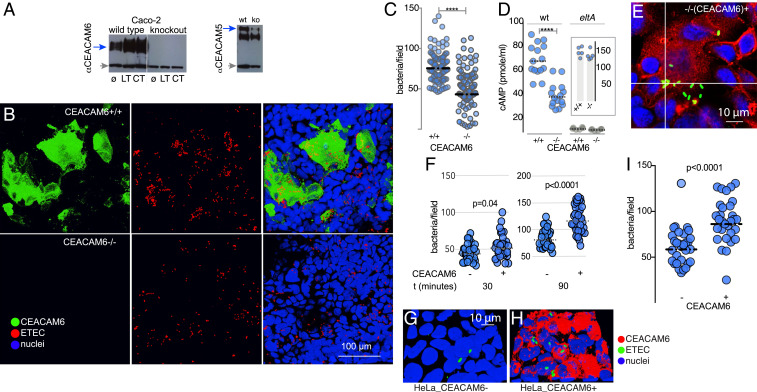
Fig. 2.
CEACAM6 facilitates ETEC pathogen–host interactions. (A) CRISPR-Cas9–generated CEACAM6−/− mutant cell line (knockout, ko) does not make CEACAM6 (anti-CEACAM6 immunoblot; Left), but makes CEACAM1 and CEACAM5 (Right). Gray arrows indicate tubulin as a loading control. ø, untreated cells; LT and CT, stimulation with heat-labile or cholera toxin overnight, respectively. (B) Confocal images of wild type H10407 ETEC adherent to CEACAM6+/+ cells (Top) and CEACAM6-deficient cells (−/−; Bottom). (C) CEACAM6 is required for optimal interaction of ETEC with intestinal epithelial cells. (D) CEACAM6 is required for efficient toxin delivery by ETEC. Intracellular cAMP concentrations were determined in CEACAM6+/+ and −/− cells following infection with either wild type (wt) or control LT-negative eltA mutant strain (n = 15 replicates from a total of three independent experiments). (Inset) cAMP responses (in pmol/mL) of CEACAM6+/+ and −/− cells following treatment with forskolin. Symbols represent n = 5 replicates (bars represent geometric mean values; ****P < 0.0001 by Mann–Whitney U two-tailed nonparametric testing). (E and F) Restoration of CEACAM6 expression (red) in CEACAM6−/− mutant cell line G2 restores ETEC (green) adhesion. Graphs depict ETEC adhesion to G2 cells (−/+CEACAM6) expressed as bacteria per field 30 and 90 min after infection. (G–I) Introduction of CEACAM6 into HeLa cells promotes ETEC adhesion.