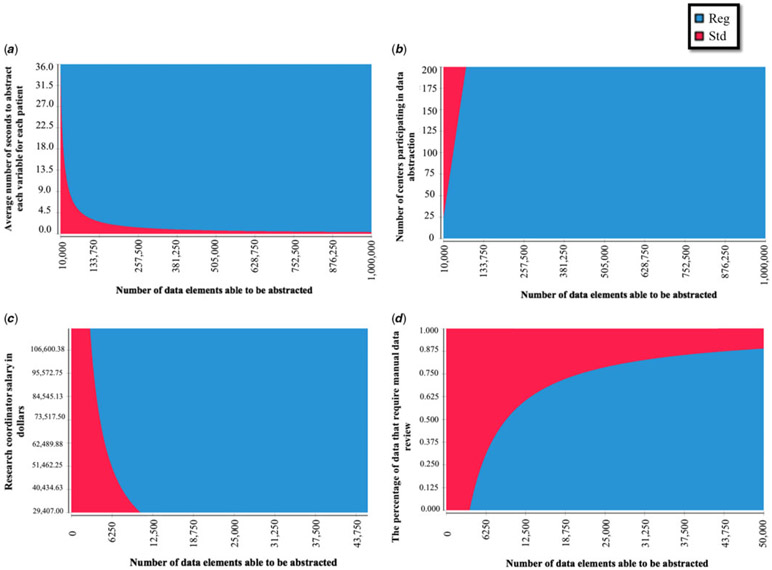
Figure 3.
Two-way sensitivity analyses. Graphs display the interactive effects on cost savings of two simultaneous changes in study parameters when comparing a standard trial design versus a registry-based trial when accessing locally held data via a study-specific data query. (a) Displays the effects of changes in the total number of data elements able to be abstracted versus the average number of seconds it takes a research coordinator to abstract each variable for each patient; (b) displays the effects of changes in the total number of data elements able to be abstracted versus the number of centres participating in data abstraction; (c) displays the effects of changes in the total number of data elements able to be abstracted versus the salaries of research coordinators; and (d) displays the effects of changes in the total number of data elements able to be abstracted versus the quality of the registry data (the percentage of data that require manual data review). Red denotes scenarios in which a standard trial design would be less costly. Blue denotes scenarios in which registry-based trials would be less costly.