Fig. 2.
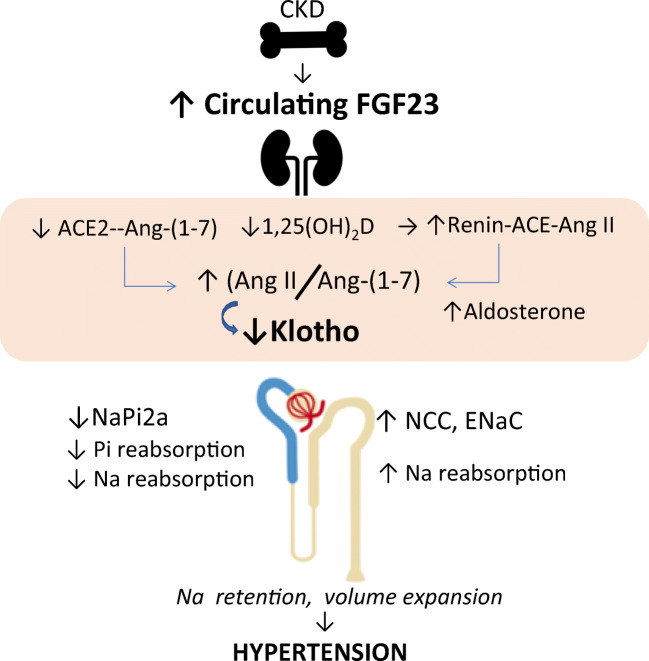
Excess FGF23 and Klotho insufficiency links to vitamin D, the RAAS, sodium homeostasis, and hypertension in CKD. Augmented FGF23 production by the bone results in elevated circulating FGF23 levels. FGF23 effects in the kidney are mediated by activation of the FGFR1/Klotho complex and result in reduced 1,25(OH)2D synthesis, upregulated renin with increased ACE/Ang II, and reduced ACE2/Ang-(1-7) formation, leading to an elevated Ang II/Ang-(1-7) ratio, which contribute to sodium retention, kidney damage, and hypertension. Membrane-bound Klotho is normally induced by 1,25(OH)2D, therefore reduced 1,25(OH)2D, and elevated Ang II (via the Ang II type 1 receptor, not shown), both suppress Klotho production. The latter contributes to FGF23 resistance further augmenting FGF23 levels. FGF23 also acts separately on both the renal proximal and the distal tubules mostly in a Klotho-dependent fashion but by distinctly different signaling systems (not shown). In the proximal tubule (blue), FGF23 inhibits the re-uptake of phosphate by regulating the sodium phosphate transporter NaPi 2a (and NaPi 2c, not shown). Since NaPi 2a transports three sodium ions together with phosphate, FGF23-mediated inhibition of this transporter causes natriuresis. In the distal tubule (light brown), FGF23 activates the NCC to increase reabsorption of sodium and ultimately promotes volume retention thus contributing to hypertension. While upregulation of the NCC favors hypertension, concurrent inhibition of the NaPi 2a may counterbalance these sodium-retentive effects on vascular homeostasis and attenuate hypertension. The reduced Klotho expression in CKD restrains the formation of the FGFR1-Klotho complex; the ensuing FGF23 resistance, and the increased circulating Ang II and aldosterone, contribute to the increment in FGF23 secretion. The higher FGF23 levels contribute to accentuate deficiency of vitamin D and ACE2, which, in turn, drive Ang II overactivity that further accentuates Klotho deficiency