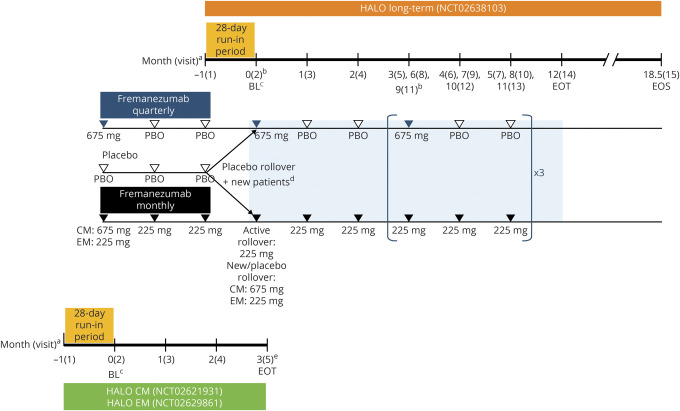
Figure 1. Study design for the long-term safety and efficacy study of fremanezumab.
This long-term study was an extension of the Efficacy and Safety of Subcutaneous Administration of Fremanezumab (TEV-48125) for the Preventive Treatment of Migraine (HALO) chronic migraine (CM) and episodic migraine (EM) studies that allowed an additional subset of new patients who had not previously participated in the HALO studies to directly enroll. BL = baseline; EOS = end of study; EOT = end of treatment; PBO = placebo. a Monthly dosing refers to dosing approximately every 4 weeks (28 days). b To maintain the study blind, all patients received 3 injections at visits 2,5, 8, and 11. A single injection was administered all other months. c Baseline refers to the 28-day run-in period (for headache variables only) and day 0 of the HALO CM or EM studies for rollover patients or to the 28-day run-in period (for headache variables only) and day 0 of this long-term study for new patients. d Rollover patients who received placebo during the HALO CM or EM studies and new patients not rolling over from one of the HALO CM or EM studies who met eligibility criteria after completing a 28-day run-in period were randomized in a 1:1 ratio on day 0 of this long-term study to receive fremanezumab quarterly at 675 mg or monthly at 225 mg (CM monthly group received a single 675 mg dose of fremanezumab at baseline). eFor patients who began this long-term study (visit 2) on the same day as the EOT visit of the HALO CM or EM study, the EOT visit procedures/assessments for the HALO CM or EM study were completed before procedures/assessments for this study began.