Abstract
Introduction:
There is still controversy about the effect of early hypothermia on the outcome of spinal cord injury (SCI). The aim of this review article is to investigate the effect of local or general hypothermia on improving the locomotion after traumatic SCI.
Methods:
Electronic databases (Medline and Embase) were searched from inception until May 7, 2018. Two independent reviewers screened and summarized the relevant experimental studies on hypothermia efficacy in traumatic SCI. The data were analyzed and the findings were presented as pooled standardized mean difference (SMD) and 95% confidence interval (95% CI).
Results:
20 papers containing 30 separate experiments were included in meta-analysis. The onset of hypothermia varied between 0 and 240 minutes after SCI. Administration of hypothermia has a positive effect on locomotion following SCI (SMD=0.56 95% CI: 0.18-0.95, p=0.004). Subgroup analysis showed that general hypothermia improves locomotion recovery (SMD =0.89, 95% CI: 0.42 to 1.36; p <0.0001), while local hypothermia does not have a significant effect on motor recovery (SMD=0.20, 95 % CI: -0.36-0.76, p=0.478). In addition, general hypothermia was found to affect motor recovery only if its duration was between 2 and 8 hours (SMD=0.89; p<0.0001) and the target temperature for induction of hypothermia was between 32 and 35° C (SMD=0.83; p<0.0001).
Conclusion:
We found that general hypothermia improves locomotion after SCI in rats. Duration of induction and the target temperature are two essential considerations for general therapeutic hypothermia.
Key Words: Spinal Cord Injuries, Hypothermia, Movement Disorders, Rats
Introduction
Current treatments that are considered to improve spinal cord injury (SCI) outcome include medicinal therapy (e.g. methylprednisolone), surgery, and rehabilitation (1-3). However, these clinical managements in SCI have not been satisfactory and even some recent studies recommended against these managements, such as application of methylprednisolone 8 hours post-SCI (2). While researchers are looking for new therapeutic interventions such as stem cell and laser therapy to postpone or stop the pathological process of SCI (4-10), hypothermia is one of the old therapeutic interventions that has been suggested in different studies. The advantages of reducing body temperature have been reported in different subjects such as cardiac arrest (11), neonatal ischemic-hypoxic encephalopathy (12, 13), hepatic encephalopathy (14), cerebral aneurysm (15), stroke (16-18), traumatic brain injury (19, 20) and SCI (21-23). For instance, in a clinical trial, Kim et al. demonstrated that prehospital induction of mild hypothermia improved survival and neurological status of cardiac arrest patients (11). In another study, Seo et al. reported that hypothermia has a neuroprotective effect and it could decrease apoptosis and autophagy after SCI (22).
Hypothermia can be classified into three groups, including severe hypothermia (below 28°C), moderate hypothermia (28 to 32°C), and mild hypothermia (33-35°C). Early studies have shown the efficacy of local hypothermia in improving neurological complications in animal models (24-27), and the effectiveness of general hypothermia, reported in subsequent studies, was far greater (28). Human studies also revealed that hypothermia reduced the complications of SCI (29-31). However, there is still substantial controversy on the effectiveness of hypothermia in controlling post-SCI complications. Some other studies failed to find a similar effect. For example, Lo et al. have demonstrated that using general hypothermia only improved locomotion within the first weeks after SCI, but ultimately, after 8 weeks of follow-up, the motor score did not differ from the control group (32). In another study, Maybhate et al. showed that general hypothermia provided a positive neuroprotective effect in acute and subacute phases of SCI and could improve hind limb locomotion (33). However, Batchelor et al. (34) and Morizane et al. (35) showed that hypothermia provided significant improvement in locomotion 8 weeks after SCI. In addition, whether the intensity of hypothermia, lesion site, the severity of injury, and other factors influence the effect of therapeutic hypothermia on improvement of SCI outcome remains widely unknown. Batchelor et al. (34) showed that hypothermia could improve locomotion if the duration of hypothermia induction was 8 hours. However, Lo et al. did not show a significantly higher post-injury locomotion after 6-hour hypothermia (36). Teh et al. (37) showed that moderate hypothermia did not have a significant effect on motor function after SCI, while Morizane et al. (35) study showed the contrary. Therefore, in the present study, we aimed to systematically analyze the effect of hypothermia on improving locomotion recovery in animal models of SCI.
Methods
Study design and search strategy
The method of searching databases and performing analyses was the same as the previously published Meta-analyzes by the present researchers (38-43). Following the selection of keywords related to SCI and hypothermia, Medline and Embase databases were searched from inception until May 7, 2018. To find additional articles, bibliography of related article and reviews was screened. Google search engine, Google Scholar and the ProQuest were also searched. The search query for Medline (via OvidSp) is provided below.
1. Spinal Cord Injury / OR Quadriplegia / OR Paraplegia / OR (Spinal Cord / AND "Wounds and Injuries") OR (("Spinal Cord" adj (Injur * OR Contus * OR Trauma * OR Posttrauma * OR Transect * OR Lacerat * OR Compromi * OR Lesion * OR Rupture *)) OR Quadripleg * OR Paraplegic * OR Tetraplegi * OR Quadripares' s OR ((Trauma * OR Posttrauma *) adj Myelopath *)) ti.ab.
2. Cold Temperature / OR Hypothermia / OR Ex Cryotherapy / OR Cryoanesthesia / OR Ex Hypothermia, Induced / OR Body Temperature Changes / OR Gastric Hypothermia / OR (Cryoan? Esthe * OR Cryogen * OR Cryotherap * OR Cryotherm * OR Cryotreat * OR Cold * OR Cool * OR Chill * OR Hypotherm * OR (Temperature adj4 (Decreas * OR Reduc * OR Low * OR Minim * OR Taper *)) OR ((Decreas * OR Reduc * OR Low * OR Minim * OR Taper *) adj4 Temperature) OR "Artificial Hybernation" OR (Refrigerat * adj An? Esthe *)) ti.ab.
3. 1 AND 2
Eligibility criteria
All rat studies that examined the effects of general or local hypothermia on locomotion recovery following traumatic SCI (contusion, compression, hemisection, transection, crush injury model) were included. Exclusion criteria consisted of in vitro studies, lack of functional assessment, lack of control group, non-traumatic SCI (aortic cross-clamping model of SCI), mild severity of SCI model, combination therapy of hypothermia with other treatments, not reporting details of hypothermia administration and reviews.
Quality assessment and Data Extraction
Two independent reviewers initially screened titles and abstracts, identified potentially relevant articles, and screened their full texts based on inclusion and exclusion criteria. They independently recorded animals’ age/weight, strain, species and sex, mechanism of the induction of SCI, details of hypothermia induction, number of samples and locomotion score. In animal studies, examination for locomotion recovery is usually conducted in several time sessions, so we only included the last session of follow-up. If the results were presented in the charts, data were extracted using the WebPlotDigitizer software. WebPlotDigitizer is a reliable software for extracting data and its accuracy has been proven in a previous study (44). Severity of injury was categorized based on the definition given in the article by Cheriyan et al. (45). Hypothermia was classified into three groups, including severe hypothermia (target temperature below 28°C), moderate hypothermia (target temperature between 28 and 32°C), and mild hypothermia (target temperature between 33 and 35 °C). Any disagreements were resolved through discussion with the third reviewer.
Qualitative assessment of papers was performed based on the method suggested in Hassannejad et al. study (46). Disagreements were resolved through discussion with the third reviewer.
Statistics
Data were analyzed using STATA software version 14.0. Locomotion score was recorded as mean and standard deviation and using the "metan" command, a random-effect analysis was performed. Finally, the output was presented as pooled standardized mean difference (SMD) and 95% confidence interval (95% CI). I2 tests were used to evaluate heterogeneity between the studies. In cases with high levels of heterogeneity (I2≥ 50%), subgroup analysis was performed to determine the cause of heterogeneity. Funnel Plot was used to identify publication bias using Egger's test (47).
Results
Studies’ characteristics
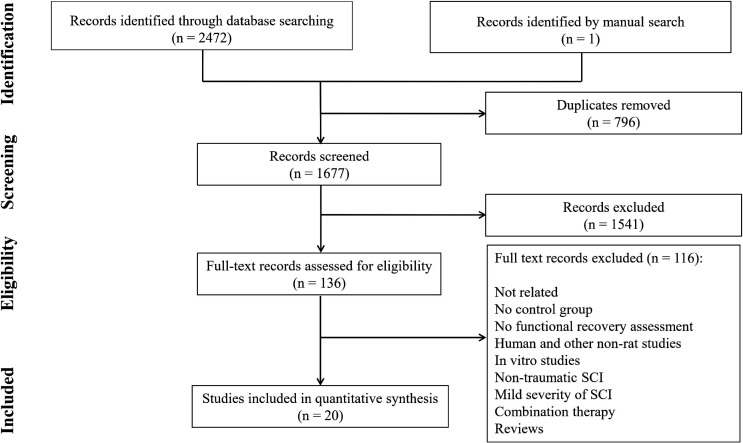
The titles and abstracts of 1677 non-duplicate articles were screened and then full text of 136 articles were selected for in-depth assessment. Finally, the data of the 20 included studies were pooled in a meta-analysis (33-37, 48-62) (Fig. 1). These studies contained 30 separate experiments. All studies used the compression / contusion model to induce spinal cord injury. Intensity of injury was moderate in 22 experiments and severe in eight experiments. Location of injury was cervical in two experiments, thoracic in 26 experiments, and in the thoracolumbar or lumbar regions in two experiments. The onset of hypothermia varied between 0 and 240 minutes after SCI (0 minutes in 11 experiments and 30 minutes in nine experiments). The duration of hypothermia also varied between 60 minutes and 2880 minutes. 15 experiments assessed the effect of local hypothermia and 15 experiments investigated the effect of general hypothermia on locomotion recovery. Table 1 shows a summary of the eligible articles.
Figure 1.
PRISMA flow diagram of the present meta-analysis
Table 1.
Characteristics of included animal studies
| Author; Year; Country | Sample size (control; treated) | Gender; Strain; Species | Injury model | Severity | Injury level | Onset of hypothermia post-injury (min) | Hypothermia duration (min) | Type of hypothermia | Cooling temperature (°C) | Follow up duration (day) |
|---|---|---|---|---|---|---|---|---|---|---|
| Barbosa; 2014; Brazil | 15; 15 | Male and female; Wistar; Rat | Contusion | Moderate | T9-T10 | 0 | 20 | Local | 25 | 42 |
| Batchelor; 2010; Australia | 36; 36 | Female ; Fischer; Rat | Contusion | Moderate | T8 | 30 | 450 | General | 33 | 56 |
| Casas; 2005; USA | 14; 42 | Female ; SD; Rat | Contusion | Moderate | T10 | 30 | 180 | Local | 24±2.3 | 42 |
| Dimar; 2000; USA | 26; 26 | Male; SD; Rat | Contusion | Moderate | T10 | 0 | 120 | Local | 19 | 35 |
| Grulova; 2013; Slovakia | 12; 4 | Male; Wistar; Rat | Compression | Moderate | T8–T9 | 0 | 198 | General | 32.0 | 28 |
| Ha; 2008; Korea | 8; 8 | Male; SD; Rat | Contusion | Severe | T9 | 0 | 2880 | Local | 30 | 7 |
| Hosier; 2015; USA | 10; 8 | Female ; Long-Evans; Rat | Contusion | Severe | C7 | 240 | 240 | General | 33.0±0.3 | 42 |
| Kao; 2011; Taiwan | 8; 8 | Male; SD; Rat | Contusion | Moderate | T8-T9 | 0 | 120 | General | 31 to 35 | 4 |
| Karamouzian; 2015; Iran | 20; 60 | Male; Wistar; Rat | Contusion | Moderate | T8–9 | 30 | 180 | General | 33.5±0.5 | 42 |
| Lo; 2009; USA | 9; 9 | Female; Fischer; Rat | Contusion | Moderate | C5 | 5 | 240 | General | 33.0±0.3 | 56 |
| Maybhate; 2012; USA | 8; 7 | Female; Lewis; Rat | Contusion | Moderate | T8 | 120 | 120 | General | 32 | 28 |
| Morizane; 2012; Japan | 10; 9 | Female ; Wistar; Rat | Contusion | Severe | T11 | 0 | 2880 | Local | 33 | 56 |
| Morochovic; 2008; Slovakia | 10; 10 | Male; SD; Rat | Compression | Severe | T8-T9 | 25 | 60 | Local | 28.5±0.3 | 28 |
| Ok; 2012; Korea | 7; 14 | Male; SD; Rat | Contusion | Severe | T9 | 0 | 2880 | Local and general | 20 | 42 |
| Seo; 2015; Korea | 5; 5 | Male; SD; Rat | Contusion | Moderate | T9 | 15 | 240 | Systemic | 30-32 | 42 |
| Teh; 2017; Singapore | 6; 11 | NR; SD; Rat | Contusion | Moderate | T8 | 120 | 300 | Local | 32±0.5 | 42 |
| Topuz; 2010; Turky | 8; 8 | Male; Wistar; Rat | Compression | Moderate | T10-T12 | 30 | 120 | Local | 27-29 | 42 |
| Westergren; 2000; Sweden | 9; 7 | Male; SD; Rat | Compression | Moderate | T7-T8 | 60 | 120 | General | 30 | 14 |
| Xu; 2016; China | 12; 12 | Male; SD; Rat | Compression | Moderate | T10 | 0 | 160 | Local | 18 | 21 |
| Yu; 2000; USA | 8; 12 | Female ; SD; Rat | Contusion | Moderate | T10 | 30 | 240 | General | 33.1 | 44 |
GMLC: GottingenMinnesotaLiběchov crossbred; SD: Sprague–Dawley
Quality assessment of articles and publication bias
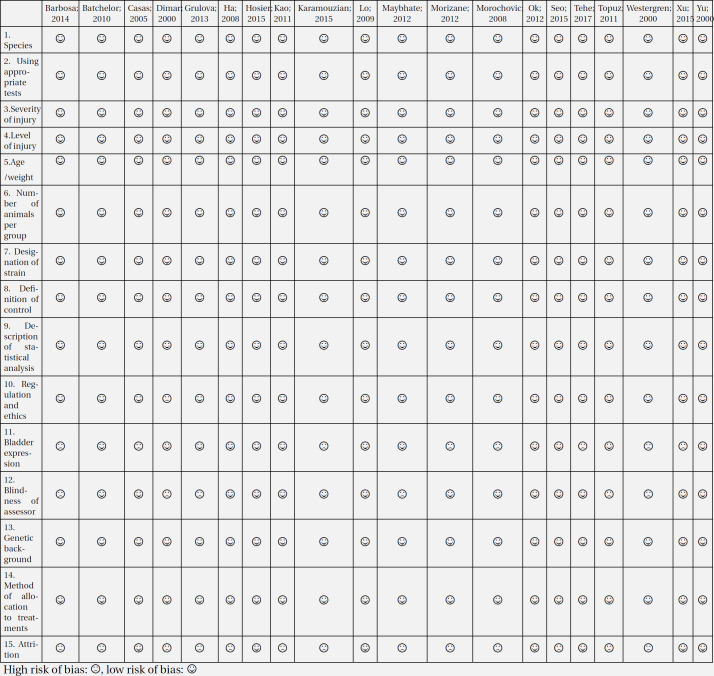
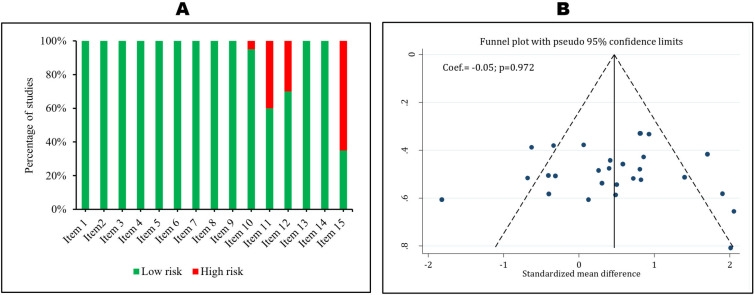
Table 2 and Fig. 2 show the quality status and risk of bias among the studies. There was no publication bias in present meta-analysis (p=0.972). Among the studies, 40.0% did not report bladder expansion and 65.0% did not describe the reasons for excluding animals from the experiment. There was one study that did not have regulation and ethical statement, and six studies that did not clearly report blinding status.
Table 2.
Quality assessment of included studies
Figure 2.
Quality assessment (A) and risk of publication bias (B) in the current meta-analysis. A) Item 1. Species; item 2. Using appropriate tests; item 3. Severity of injury; item 4. Level of injury; item 5. Age/weight; item 6. Number of animals per group; item 7. Designation of strain; item 8. Definition of control; item 9. Description of statistical analysis; item 10. Regulation and ethics; item 11. Bladder expression; item 12. Blindness of assessor; item 13. Genetic background; item 14. Method of allocation to treatments; item 15. Description of the reasons for excluding animals from the experiment during the study. B) There is no publication bias in the present meta-analysis
The effect of hypothermia on locomotion recovery
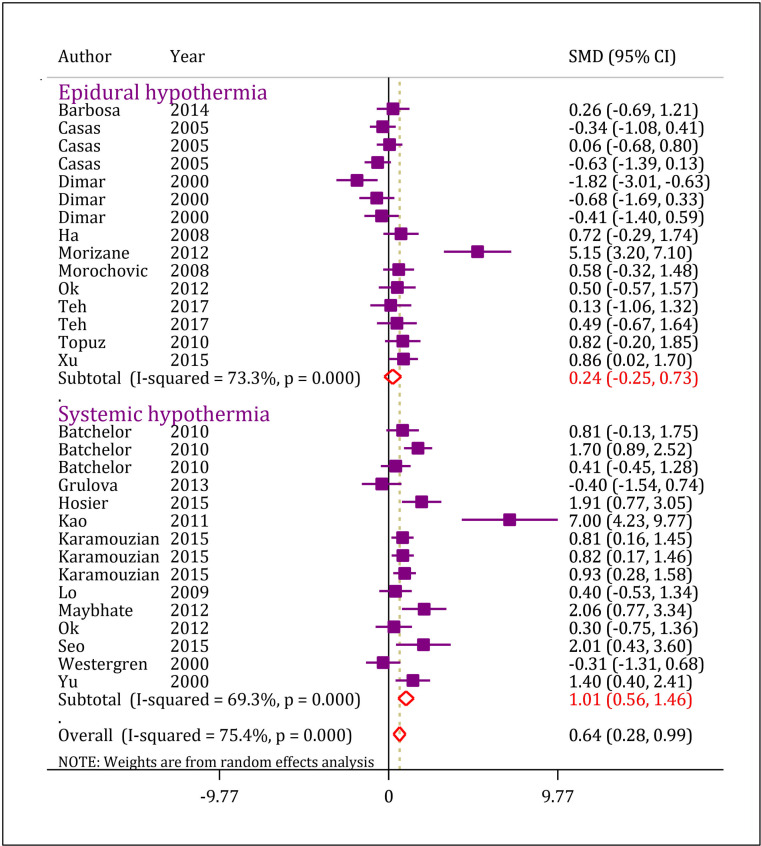
The findings of this meta-analysis showed that hypothermia (local and general) has a positive effect on locomotion following SCI (Fig. 3) (SMD = 0.64, 95% CI: 0.28 to 0.99, p < 0.0001, I2 = 75.4%, p <0.0001). Subgroup analysis showed that general hypothermia improves locomotion (SMD = 1.01, 95% CI: 0.56 to 1.46; p <0.0001), while local hypothermia does not have any effect on motor recovery (SMD = 0.24, 95% CI: -0.25 to 0.73; p = 0.341).
Figure 3.
Forest plot of general and local hypothermia on motor function recovery after spinal cord injury. Data were extracted from 20 studies including 30 separate experiments. CI: Confidence interval; SMD: Standardized mean difference
Subgroup analysis of general hypothermia on SCI
The analysis showed that general hypothermia only improves locomotion in moderate injuries (SMD = 1.00, p < 0.0001), whereas in severe injuries, does not have a significant effect (SMD = 1.09; p = 0.173). Level of injury was another factor affecting the influence of general hypothermia. The effect of general hypothermia on locomotion recovery was significant only in case of thoracic injuries (SMD = 1.00; p < 0.0001). In addition, it was found that general hypothermia could only improve locomotion if its duration was between 2 and 8 hours (SMD = 0.93; p < 0.0001), but when the duration was less than 2 hours (p = 0.102) or more than 8 hours (p = 0.572), its effect was not significant.
Another factor influencing the effectiveness of general hypothermia was the intensity of hypothermia. Analysis demonstrated that induction of mild general hypothermia (SMD = 0.95; p <0.0001) resulted in motor recovery improvement, while moderate (p = 0.380) and severe (p = 0.571) general hypothermia had no effect on locomotion recovery. Another factor influencing locomotion recovery was the duration of follow-up. Analyses showed that the role of general hypothermia in improving locomotion recovery is significant only when the animals were followed up for at least 4 weeks (SMD for 4 to 7 weeks follow-up = 1.00, p < 0.0001; SMD for 8 weeks and more follow-up = 0.85, p = 0.003) (Table 3).
Table 3.
Subgroup analysis for assessment of general hypothermia on motor function recovery
| Subgroups | Number of experiments | Effect size | Heterogeneity (p value) | |
|---|---|---|---|---|
| SMD (95% CI) | p value | |||
| Severity of injury* | ||||
| Moderate | 13 | 1.00 (0.51 to 1.49) | <0.001 | 71.0% (<0.0001) |
| Severe | 2 | 1.09 (-0.48 to 2.66) | 0.173 | 75.5% (0.043) |
| Level of injury | ||||
| Cervical | 2 | 1.12 (-0.36 to 2.59) | 0.137 | 75.0% (0.046) |
| Thoracic | 13 | 1.00 (0.51 to 1.49) | <0.001 | 71.1% (<0.0001) |
| Onset of hypothermia after SCI | ||||
| 1 hour or less | 11 | 0.92 (0.31 to 1.52) | 0.003 | 74.3% (<0.0001) |
| More than 1 hour | 4 | 1.22 (0.66 to 1.78) | <0.0001 | 40.9% (0.166) |
| Duration of hypothermia | ||||
| 2 hours or less | 3 | 2.64 (-0.52 to 5.80) | 0.102 | 92.7% (<0.0001) |
| 2 to 8 hours | 11 | 0.93 (0.58 to 1.23) | <0.0001 | 41.2% (0.074) |
| More than 8 hours | 1 | 0.30 (-0.75 to 1.36) | 0.572 | 0.0% (>0.999) |
| Intensity of hypothermia | ||||
| Mild (32 to 35 °C) | 12 | 0.95 (0.60 to 1.31) | <0.0001 | 45.0% (0.052) |
| Moderate (28 to 31.9 °C) | 2 | 2.66 (-0.76 to 6.09) | 0.380 | 92.4% (<0.0001) |
| Severe (less than 28 °C) | 1 | 0.30 (-0.75 to 1.36) | 0.571 | 0.0% (>0.999) |
| Follow up duration | ||||
| Less than 4 weeks | 2 | 3.22 (-3.94 to 10.38) | 0.377 | 95.8% (<0.0001) |
| 4 to 7 weeks | 9 | 1.00 (0.57 to 1.43) | <0.0001 | 47.4% (0.055) |
| 8 weeks and more | 4 | 0.85 (0.22 to 1.48) | 0.008 | 50.4% (0.109) |
*, Severity of injury was categorized based on the definition given in the article by Cheriyan et al (45).
CI: Confidence interval; SMD: Standardized mean difference; SCI: Spinal cord injury
Discussion
The present study showed that general hypothermia has a positive effect on locomotion recovery following SCI, but local hypothermia did not have any effect on locomotion recovery. General hypothermia's effectiveness in improving motor recovery can be attributed to its angiogenic, neurogenic, and anti-inflammatory effects (53). Following general hypothermia, microglial proliferation, TNF-α production, and neutrophil migration are significantly decreased (53, 63). However, local hypothermia reduces the survival of the grey matter in the spinal cord (48). Direct cold contact decreases perfusion of the injured spinal cord (48, 64), which increases cell mortality and cannot have protective effects.
In a meta-analysis, Batchelor et al. (2013) have demonstrated that general and local hypothermia can improve animal locomotion by about 24.5% and 26.2%, respectively, (28). However, the present study shows that local hypothermia does not have an effect on locomotion recovery after SCI. The reason for this discrepancy could be the inclusion of a variety of species, such as primates, in Batchelor's systematic review (Batchelor review). In addition, details of effect size calculation, the included articles, and quantitative control of the eligible studies were not provided in that review. Moreover, the results of neurological assessments were pooled with locomotion scores, which may result in considerable heterogeneity and possible bias. In Batchelor review, when the analysis was limited to locomotion (BBB test), it was found that the effectiveness of local hypothermia was only 8.8% (95% CI: 0.06 to 16.7%). Therefore, in that review, local hypothermia has had limited effect on post-SCI locomotion recovery. In 2016, Alkabie et al. performed a systematic review on articles from the Medline database, and evaluated the role of hypothermia on traumatic SCI outcome in animal studies. Their findings showed that hypothermia could improve locomotion recovery in animals. However, only searching in MEDLINE and not performing a meta-analysis and subgroup analysis were the greatest weaknesses of the study (65).
One of the most important findings of the present study is the effectiveness of mild general hypothermia in improving locomotion recovery. This finding is completely in discrepancy with the Batchelor review, which showed that the highest efficacy was observed when hypothermia was induced at 4 to 19°C. The reason for this controversy can be the difference in the type of analyses. Analyses of Batchelor et al. were limited to the local hypothermia (28), while we also performed analysis on general hypothermia. The results of a systematic review on human studies (level IV of evidence) was consistent with our findings, showing that mild general hypothermia is a safe method with improved outcomes of SCI (66). It seems that in severe and moderate general hypothermia, the spinal cord blood flow is reduced, with more destructive, rather than protective, effects leading to an increase in death of residual spinal cord cells (67).
Treatment duration was another factor influencing the effectiveness of general hypothermia. Our findings showed that if the treatment duration was less than 2 hours or more than 8 hours, general hypothermia had no effect on the outcome of SCI, which perhaps suggested a role of a therapeutic window in the effectiveness of an intervention. It is likely that less than 2 hours of induction of hypothermia is not sufficient for the researchers to observe its effects on locomotion recovery, whereas treatment for more than 8 hours may increase the destructive effects of hypothermia on motor function.
One of the limitations of the present study was the existence of significant heterogeneity between studies. Although subgroup analysis was performed, in some cases the source of heterogeneity was not recognized. Also, the duration of follow-up varied between studies, which could have had affected the findings. Subgroup analysis showed that the role of hypothermia in recovery was significant only when the animals were followed for at least 4 weeks.
Conclusion
We found that general hypothermia improves locomotion after SCI in rat models. Duration of general hypothermia being between 2 and 8 hours, and hypothermia being mild (the target temperature being 32 to 35°C) are two essential considerations for best results in general therapeutic hypothermia.
Acknowledgment
None.
Competing Interests
The authors declare that they have no competing interests.
Funding
This research has been supported by the Iran Ministry of Health and Medical Education and Tehran University of Medical Sciences & Health Services grant (ID number: 96-04-159-36946).
Conflict of Interest
Prof. Alex R. Vaccaro has potential conflict of interest. The conflict of interest statement is presented in Table S1. Other authors declared that there is no conflict of interest.
Table S1.
The conflict of interest statement of Prof. Alex R. Vaccaro. Health care entity relationships and investments
| Entity | Relationship (see legend below) |
|---|---|
| Replication Medica | d |
| Medtronics | c |
| Stryker Spine | c, |
| Globus | c,d |
| Paradigm Spine | d |
| Stout Medical | d |
| Progressive Spinal Technologies | d |
| Advanced Spinal Intellectual Properties | d |
| Aesculap | c |
| Spine Medica | d |
| Computational Biodynamics | d |
| Spinology | d |
| Flagship Surgical | d |
| Cytonics | d |
| Bonovo Orthopaedics | d |
| Electrocore | d |
| Insight Therapeutics | d |
| FlowPharma | d |
| Rothman Institute and Related Properties | d |
| AO Spine | g |
| Innovative Surgical Design | d |
| Orthobullets | d |
| Thieme | c |
| Jaypee | c |
| Elseviere | c |
| Taylor Francis/Hodder and Stoughton | c |
| Expert testimony | g |
| Vertiflex | d |
| Avaz Surgical | d |
| Dimension Orthotics, LLC | d |
| SpineWave | c |
| Atlas Spine | c |
| Nuvasive | d |
| Parvizi Surgical Innovation | d |
| Franklin Bioscience | d |
| Deep Health | d |
a. Consulting / Independent Contractor
b. Service on Scientific Advisory Board / Board of Directors / Service on Committees
c. Receipt of Royalty Payments
d. Stock / Stock Option Ownership Interests
e. Institutional / Educational Grant
f. Deputy editor/ Editor/Editorial Board
g. Member in good standing// Independent Contractor
Author contribution
Study design: VRM, ARV, MY and MH
Conducting the search: FS
Data collection: MY, MHVM and LH
Analysis: MH and MY
Interpreting of the findings: VRM and ARV
Drafting: MY, MHVM and LH
Critically revised the paper: All authors
Authors ORCID:
Mahmoud Yousefifard: 0000-0001-5181-4985
Mohammad Hossein Vazirizadeh-Mahabadi: 0000-0003-2435-9985
Leila Haghani: 0000-0002-8649-2485
Farhad Shokraneh: 0000-0001-9687-8560
Alexander R. Vaccaro: 0000-0002-8073-0796
Vafa Rahimi-Movaghar: 0000-0001-7347-8767
Mostafa Hosseini: 0000-0002-1334-246X
References
- 1.Yousefifard M, Rahimi-Movaghar V, Baikpour M, Ghelichkhani P, Hosseini M, Jafari A, et al. Early versus late spinal decompression surgery in treatment of traumatic spinal cord injuries; a systematic review and meta-analysis. Emergency. 2017;5(1) [PMC free article] [PubMed] [Google Scholar]
- 2.Evaniew N, Belley-Côté EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylprednisolone for the treatment of patients with acute spinal cord injuries: A systematic review and meta-analysis. Journal of neurotrauma. 2016;33(5):468–81. doi: 10.1089/neu.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnuson DS, Dietrich WD. Introduction to the Special Issue on Locomotor Rehabilitation after Spinal Cord Injury. Journal of Neurotrauma. 2017;34(9):1711–2. doi: 10.1089/neu.2017.5126. [DOI] [PubMed] [Google Scholar]
- 4.Janzadeh A, Sarveazad A, Yousefifard M, Dameni S, Samani FS, Mokhtarian K, et al. Combine effect of Chondroitinase ABC and low level laser (660nm) on spinal cord injury model in adult male rats. Neuropeptides. 2017 doi: 10.1016/j.npep.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Mojarad N, Janzadeh A, Yousefifard M, Nasirinezhad F. The role of low level laser therapy on neuropathic pain relief and interleukin-6 expression following spinal cord injury: An experimental study. Journal of chemical neuroanatomy. 2017 doi: 10.1016/j.jchemneu.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Sarveazad A, Babahajian A, Bakhtiari M, Soleimani M, Behnam B, Yari A, et al. The combined application of human adipose derived stem cells and Chondroitinase ABC in treatment of a spinal cord injury model. Neuropeptides. 2017;61:39–47. doi: 10.1016/j.npep.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Yousefifard M, Nasirinezhad F, Shardi Manaheji H, Janzadeh A, Hosseini M, Keshavarz M. Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem cell research & therapy. 2016;7:36. doi: 10.1186/s13287-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience. 2016;322:377–97. doi: 10.1016/j.neuroscience.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini M, Karami Z, Janzadenh A, Jameie SB, Haji Mashhadi Z, Yousefifard M, et al. The Effect of Intrathecal Administration of Muscimol on Modulation of Neuropathic Pain Symptoms Resulting from Spinal Cord Injury; an Experimental Study. Emergency (Tehran, Iran) 2014;2(4):151–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Nasirinezhad F, Hosseini M, Karami Z, Yousefifard M, Janzadeh A. Spinal 5-HT3 receptor mediates nociceptive effect on central neuropathic pain; possible therapeutic role for tropisetron. The journal of spinal cord medicine. 2016;39(2):212–9. doi: 10.1179/2045772315Y.0000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. Jama. 2014;311(1):45–52. doi: 10.1001/jama.2013.282173. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. The Journal of pediatrics. 2013;163(2):465–70. doi: 10.1016/j.jpeds.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Reinboth BS, Köster C, Abberger H, Prager S, Bendix I, Felderhoff-Müser U, et al. Endogenous hypothermic response to hypoxia reduces brain injury: implications for modeling hypoxic-ischemic encephalopathy and therapeutic hypothermia in neonatal mice. Experimental neurology. 2016;283:264–75. doi: 10.1016/j.expneurol.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Karvellas CJ, Todd Stravitz R, Battenhouse H, Lee WM, Schilsky ML. Therapeutic hypothermia in acute liver failure: a multicenter retrospective cohort analysis. Liver Transplantation. 2015;21(1):4–12. doi: 10.1002/lt.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karibe H, Sato K, Shimizu H, Tominaga T, Koshu K, Yoshimoto T. Intraoperative mild hypothermia ameliorates postoperative cerebral blood flow impairment in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2000;47(3):594–601. [PubMed] [Google Scholar]
- 16.Chen J, Zhang Z, Liu J, Zhou R, Zheng X, Chen T, et al. Acellular spinal cord scaffold seeded with bone marrow stromal cells protects tissue and promotes functional recovery in spinal cord-injured rats. J Neurosci Res. 2014;92(3):307–17. doi: 10.1002/jnr.23311. [DOI] [PubMed] [Google Scholar]
- 17.Hong JM, Lee JS, Song HJ, Jeong HS, Choi HA, Lee K. Therapeutic hypothermia after recanalization in patients with acute ischemic stroke. Stroke. 2014;45(1):134–40. doi: 10.1161/STROKEAHA.113.003143. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, Sun J, Li J, Wang L, Hall CL, Dix TA, et al. Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice. Neuroscience. 2013;252:489–500. doi: 10.1016/j.neuroscience.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. The New England journal of medicine. 2015;373(25):2403–12. doi: 10.1056/NEJMoa1507581. [DOI] [PubMed] [Google Scholar]
- 20.Beca J, McSharry B, Erickson S, Yung M, Schibler A, Slater A, et al. Hypothermia for Traumatic Brain Injury in Children-A Phase II Randomized Controlled Trial. Critical care medicine. 2015;43(7):1458–66. doi: 10.1097/CCM.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Pearse DD. Therapeutic Hypothermia in Spinal Cord Injury: The Status of Its Use and Open Questions. International journal of molecular sciences. 2015;16(8):16848–79. doi: 10.3390/ijms160816848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo JY, Kim YH, Kim JW, Kim SI, Ha KY. Effects of Therapeutic Hypothermia on Apoptosis and Autophagy After Spinal Cord Injury in Rats. Spine. 2015;40(12):883–90. doi: 10.1097/BRS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Zhang J. Effects of hypothermia combined with neural stem cell transplantation on recovery of neurological function in rats with spinal cord injury. Molecular medicine reports. 2015;11(3):1759–67. doi: 10.3892/mmr.2014.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albin MS, White RJ, Acosta-Rua G, Yashon D. Study of functional recovery produced by delayed localized cooling after spinal cord injury in primates. Journal of Neurosurgery. 1968;29(2):113–20. doi: 10.3171/jns.1968.29.2.0113. [DOI] [PubMed] [Google Scholar]
- 25.Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. Journal of neurosurgery. 1969;30(6):693–7. doi: 10.3171/jns.1969.30.6.0693. [DOI] [PubMed] [Google Scholar]
- 26.Salzano RP, Ellison LH, Altonji PF, Richter J, Deckers PJ. Regional deep hypothermia of the spinal cord protects against ischemic injury during thoracic aortic cross-clamping. The Annals of thoracic surgery. 1994;57(1):65–71. doi: 10.1016/0003-4975(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 27.Rokkas CK, Cronin CS, Nitta T, Helfrich LR, Lobner DC, Choi DW, et al. Profound systemic hypothermia inhibits the release of neurotransmitter amino acids in spinal cord ischemia. The Journal of thoracic and cardiovascular surgery. 1995;110(1):27–35. doi: 10.1016/S0022-5223(05)80006-6. [DOI] [PubMed] [Google Scholar]
- 28.Batchelor PE, Skeers P, Antonic A, Wills TE, Howells DW, Macleod MR, et al. Systematic review and meta-analysis of therapeutic hypothermia in animal models of spinal cord injury. PloS one. 2013;8(8):e71317. doi: 10.1371/journal.pone.0071317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dididze M, Green B, Dietrich WD, Vanni S, Wang M, Levi A. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal cord. 2013;51(5):395. doi: 10.1038/sc.2012.161. [DOI] [PubMed] [Google Scholar]
- 30.Cappuccino A, Bisson LJ, Carpenter B, Snyder K, Cappuccino H. Systemic Hypothermia as Treatment for an Acute Cervical Spinal Cord Injury in a Professional Football Player: 9-Year Follow-Up. American journal of orthopedics (Belle Mead, NJ) 2017;46(2):E79–e82. [PubMed] [Google Scholar]
- 31.Levi AD, Casella G, Green BA, Dietrich WD, Vanni S, Jagid J, et al. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery. 2010;66(4):670–7. doi: 10.1227/01.NEU.0000367557.77973.5F. [DOI] [PubMed] [Google Scholar]
- 32.Lo TP, Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. Journal of Comparative Neurology. 2009;514(5):433–48. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- 33.Maybhate A, Hu C, Bazley FA, Yu Q, Thakor NV, Kerr CL, et al. Potential long-term benefits of acute hypothermia after spinal cord injury: assessments with somatosensory-evoked potentials. Critical care medicine. 2012;40(2):573–9. doi: 10.1097/CCM.0b013e318232d97e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batchelor PE, Kerr NF, Gatt AM, Aleksoska E, Cox SF, Ghasem-Zadeh A, et al. Hypothermia prior to decompression: buying time for treatment of acute spinal cord injury. Journal of Neurotrauma. 2010;27(8):1357–68. doi: 10.1089/neu.2010.1360. [DOI] [PubMed] [Google Scholar]
- 35.Morizane K, Ogata T, Morino T, Horiuchi H, Yamaoka G, Hino M, et al. A novel thermoelectric cooling device using Peltier modules for inducing local hypothermia of the spinal cord: the effect of local electrically controlled cooling for the treatment of spinal cord injuries in conscious rats. Neuroscience Research. 2012;72(3):279–82. doi: 10.1016/j.neures.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Lo TP Jr, Cho KS, Garg MS, Lynch MP, Marcillo AE, Koivisto DL, et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. Journal of Comparative Neurology. 2009;514(5):433–48. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- 37.Teh DBL, Chua SM, Prasad A, Kakkos I, Jiang W, Yue M, et al. Neuroprotective assessment of prolonged local hypothermia post contusive spinal cord injury in rodent model. Spine Journal: Official Journal of the North American Spine Society. 2018;18(3):507–14. doi: 10.1016/j.spinee.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 38.Yousefifard M, Movaghar VR, Baikpour M, Ghelichkhani P, Hosseini M, Jafari AM, et al. Early versus Late Decompression for Traumatic Spinal Cord Injuries; a Systematic Review and Meta-analysis. Emergency. 2016:4. [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini M, Yousefifard M, Aziznejad H, Nasirinezhad F. The Effect of Bone Marrow–Derived Mesenchymal Stem Cell Transplantation on Allodynia and Hyperalgesia in Neuropathic Animals: A Systematic Review with Meta-Analysis. Biology of Blood and Marrow Transplantation. 2015;21(9):1537–44. doi: 10.1016/j.bbmt.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Mirzay Razaz J, Hosseini M. Diagnostic Value of Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Detection of Pediatric Acute Kidney Injury; a Systematic Review and Meta-Analysis. International Journal of Pediatrics. 2016;4(11):3875–95. [Google Scholar]
- 41.Izadi A, Yousefifard M, Nakhjavan-Shahraki B, Baikpour M, Mirzay Razaz J, Ataei N, et al. Value of Plasma/Serum Neutrophil Gelatinase-Associated Lipocalin in Detection of Pediatric Acute Kidney Injury; a Systematic Review and Meta-Analysis. International Journal of Pediatrics. 2016;4(11):3815–36. [Google Scholar]
- 42.Hassanzadeh‐Rad A, Yousefifard M, Katal S, Asady H, Fard‐Esfahani A, Moghadas Jafari A, et al. The value of 18F‐fluorodeoxyglucose positron emission tomography for prediction of treatment response in gastrointestinal stromal tumors: a systematic review and meta‐analysis. Journal of gastroenterology and hepatology. 2016;31(5):929–35. doi: 10.1111/jgh.13247. [DOI] [PubMed] [Google Scholar]
- 43.Nakhjavan-Shahraki B, Yousefifard M, Oraii A, Sarveazad A, Hosseini M. Meta-analysis of neuron specific enolase in predicting pediatric brain injury outcomes. EXCLI J. 2017;16:995–1008. doi: 10.17179/excli2017-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drevon D, Fursa SR, Malcolm AL. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behavior modification. 2017;41(2):323–39. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 45.Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, et al. Spinal cord injury models: a review. Spinal Cord. 2014;52(8):588–95. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 46.Hassannejad Z, Sharif-Alhoseini M, Shakouri-Motlagh A, Vahedi F, Zadegan SA, Mokhatab M, et al. Potential variables affecting the quality of animal studies regarding pathophysiology of traumatic spinal cord injuries. Spinal Cord. 2016;54(8):579–83. doi: 10.1038/sc.2015.215. [DOI] [PubMed] [Google Scholar]
- 47.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casas CE, Herrera LP, Prusmack C, Ruenes G, Marcillo A, Guest JD. Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. Journal of Neurosurgery Spine. 2005;2(3):308–18. doi: 10.3171/spi.2005.2.3.0308. [DOI] [PubMed] [Google Scholar]
- 49.Dimar JR, 2nd , Shields CB, Zhang YP, Burke DA, Raque GH, Glassman SD. The role of directly applied hypothermia in spinal cord injury. Spine. 2000;25(18):2294–302. doi: 10.1097/00007632-200009150-00006. [DOI] [PubMed] [Google Scholar]
- 50.Grulova I, Slovinska L, Nagyova M, Cizek M, Cizkova D. The effect of hypothermia on sensory-motor function and tissue sparing after spinal cord injury [Erratum appears in Spine J 2014 Mar 1;14(3):A8] Spine Journal: Official Journal of the North American Spine Society. 2013;13(12):1881–91. doi: 10.1016/j.spinee.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 51.Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine. 2008;33(19):2059–65. doi: 10.1097/BRS.0b013e31818018f6. [DOI] [PubMed] [Google Scholar]
- 52.Hosier H, Peterson D, Tsymbalyuk O, Keledjian K, Smith BR, Ivanova S, et al. A Direct Comparison of Three Clinically Relevant Treatments in a Rat Model of Cervical Spinal Cord Injury. Journal of Neurotrauma. 2015;32(21):1633–44. doi: 10.1089/neu.2015.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kao CH, Chio CC, Lin MT, Yeh CH. Body cooling ameliorating spinal cord injury may be neurogenesis-, anti-inflammation- and angiogenesis-associated in rats. Journal of Trauma-Injury Infection & Critical Care. 2011;70(4):885–93. doi: 10.1097/TA.0b013e3181e7456d. [DOI] [PubMed] [Google Scholar]
- 54.Karamouzian S, Akhtarshomar S, Saied A, Gholamhoseinian A. Effects of methylprednisolone on neuroprotective effects of delay hypothermia on spinal cord injury in rat. Asian Spine Journal. 2015;9(1):1–6. doi: 10.4184/asj.2015.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morochovic R, Chuda M, Talanova J, Cibur P, Kitka M, Vanicky I. Local transcutaneous cooling of the spinal cord in the rat: effects on long-term outcomes after compression spinal cord injury. International Journal of Neuroscience. 2008;118(4):555–68. doi: 10.1080/00207450601123456. [DOI] [PubMed] [Google Scholar]
- 56.Ok JH, Kim YH, Ha KY. Neuroprotective effects of hypothermia after spinal cord injury in rats: comparative study between epidural hypothermia and systemic hypothermia. Spine. 2012;37(25):E1551–9. doi: 10.1097/BRS.0b013e31826ff7f1. [DOI] [PubMed] [Google Scholar]
- 57.Westergren H, Farooque M, Olsson Y, Holtz A. Motor function changes in the rat following severe spinal cord injury Does treatment with moderate systemic hypothermia improve functional outcome? Acta Neurochirurgica. 2000;142(5):567–73. doi: 10.1007/s007010050471. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Li N, Zhu L, Zhou Y, Cheng H. Beneficial effects of local profound hypothermia and the possible mechanism after experimental spinal cord injury in rats. Journal of Spinal Cord Medicine. 2016;39(2):220–8. doi: 10.1179/2045772315Y.0000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu CG, Jimenez O, Marcillo AE, Weider B, Bangerter K, Dietrich WD, et al. Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. Journal of Neurosurgery. 2000;93(1 Suppl):85–93. doi: 10.3171/spi.2000.93.1.0085. [DOI] [PubMed] [Google Scholar]
- 60.Barbosa MO, Cristante AF, Santos GB, Ferreira R, Marcon RM, Barros Filho TE. Neuroprotective effect of epidural hypothermia after spinal cord lesion in rats. Clinics (Sao Paulo, Brazil) 2014;69(8):559–64. doi: 10.6061/clinics/2014(08)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo JY, Kim YH, Kim JW, Kim SI, Ha KY. Effects of Therapeutic Hypothermia on Apoptosis and Autophagy After Spinal Cord Injury in Rats. Spine. 2015;40(12):883–90. doi: 10.1097/BRS.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 62.Topuz K, Colak A, Cemil B, Kutlay M, Demircan MN, Simsek H, et al. Combined hyperbaric oxygen and hypothermia treatment on oxidative stress parameters after spinal cord injury: an experimental study. Archives of Medical Research. 2010;41(7):506–12. doi: 10.1016/j.arcmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Morino T, Ogata T, Takeba J, Yamamoto H. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal cord. 2008;46(6):425. doi: 10.1038/sj.sc.3102163. [DOI] [PubMed] [Google Scholar]
- 64.Hansebout RR, Lamont RN, Kamath MV. The effects of local cooling on canine spinal cord blood flow. Canadian journal of neurological sciences. 1985;12(2):83–7. doi: 10.1017/s0317167100046758. [DOI] [PubMed] [Google Scholar]
- 65.Alkabie S, Boileau AJ. The Role of Therapeutic Hypothermia After Traumatic Spinal Cord Injury--A Systematic Review. World neurosurgery. 2016;86:432–49. doi: 10.1016/j.wneu.2015.09.079. [DOI] [PubMed] [Google Scholar]
- 66.O’Toole JE, Wang MC, Kaiser MG. Hypothermia and human spinal cord injury: updated position statement and evidence based recommendations from the AANS/CNS Joint Section on Disorders of the Spine Peripheral Nerves. 2014 [Google Scholar]
- 67.Westergren H, Farooque M, Olsson Y, Holtz A. Spinal cord blood flow changes following systemic hypothermia and spinal cord compression injury: an experimental study in the rat using Laser-Doppler flowmetry. Spinal cord. 2001;39(2):74. doi: 10.1038/sj.sc.3101127. [DOI] [PubMed] [Google Scholar]