Figure 1.
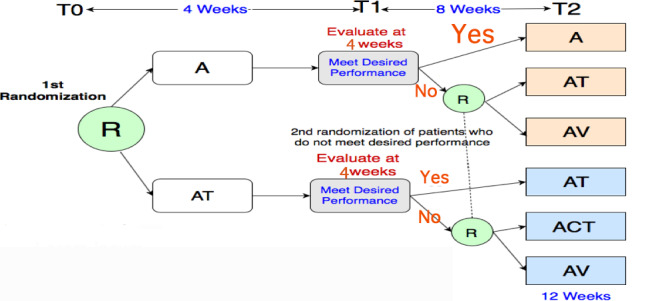
Study design. The participants are randomized first at R: one group uses only the app (A) and another group is given the app with medication information text (AT). At time point 1 (T1), after 4 weeks, both groups are evaluated for information recall (>80%). Then, after a second randomization (R), the participants are divided into the following groups: app with medication information text (AT), app with contextual medication information text (ACT), and app with visualization (AV). T0: baseline; T2: time point 2 (after 12 weeks).
