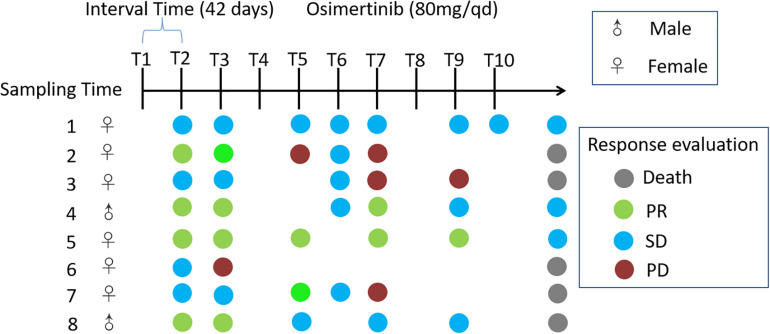
FIGURE 1.
Timeline representing the clinical course of eight patients. Key time points include timing of fecal sampling; sampling time was ranged from April 2017 to May 2018; Response evaluations include partial response (PR), stable disease (SD), progression disease (PD) and death. Response evaluations were due to December 2018. Daily dosage of osimertinib was administered orally as one 80 mg tablet.