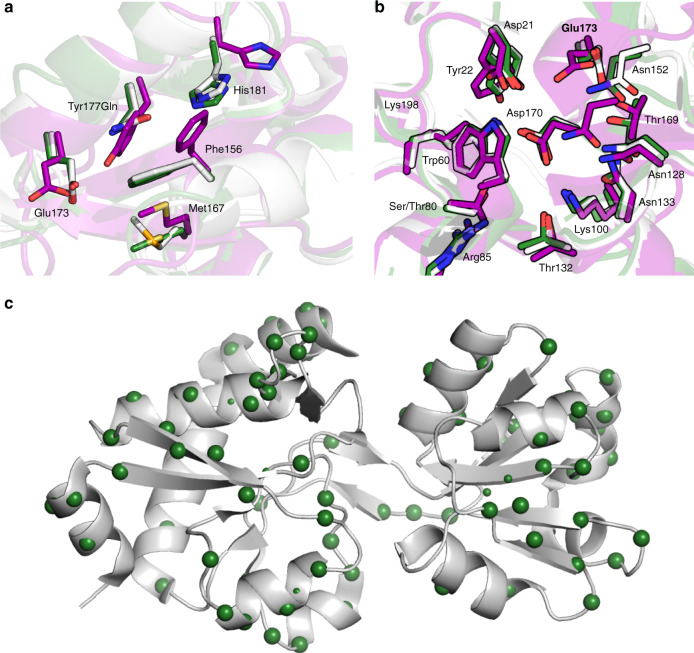
Fig. 2. Crystal structures of AncCDT-3/P188, AncCDT-5 and PaCDT.
a Structural overlay of the small domains of AncCDT-3/L188 (purple, PDB 5JOS), AncCDT-5 (green) and PaCDT (white, PDB 3KBR). The Tyr177Gln substitution that occurs between AncCDT-3 and AncCDT-5 contributes to the repositioning of Glu173. b Structural alignment of the active sites of AncCDT-3/L188 (purple, PDB 5JOS), AncCDT-5 (green) and PaCDT (PDB 3KBR, white). The small domain (residues 99–194) and large domain (residues 1–98 and 195–236) of AncCDT-3/L188 and AncCDT-5 were individually aligned with the corresponding domains of PaCDT. The sidechains of the 15 inner-shell residues identified by Clifton et al.40 are shown as sticks (Gly131 not shown), highlighting identical active site residues and side-chain conformations between AncCDT-5 and PaCDT. The HEPES molecules present in the crystal structures are omitted for clarity. c The locations of the substitutions (green spheres) between AncCDT-5 and PaCDT are shown projected onto the structure of PaCDT (PDB 3KBR).