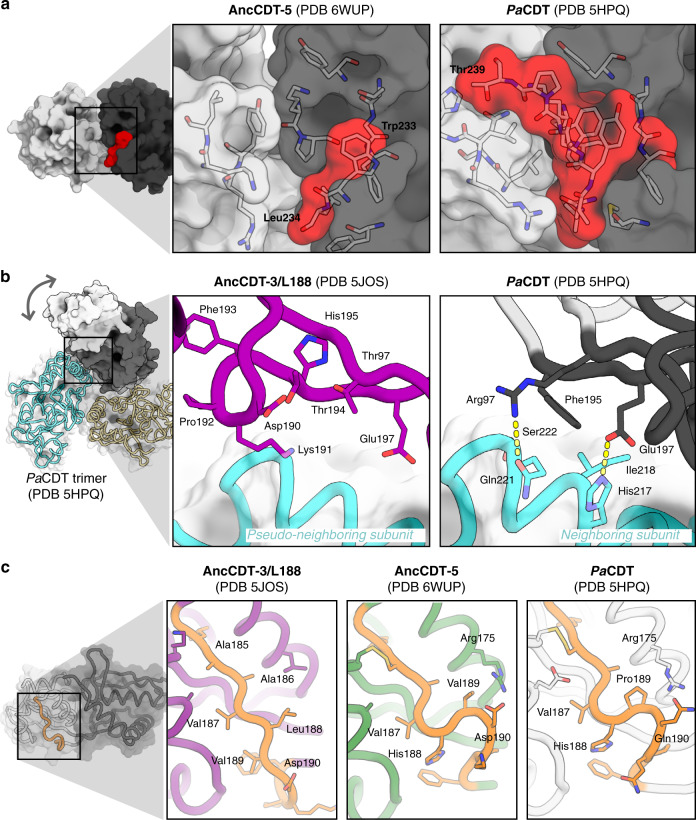
Fig. 5. Structural basis for the change in conformational sampling.
a Comparison of the crystal structures of AncCDT-5 (left) and PaCDT (5HPQ, right) highlight how the C-terminal extension (residues beyond 232 are highlighted in red) in PaCDT facilitates additional interactions between the small (white) and large (grey) domains. b PaCDT is a trimer in solution, and analysis of the crystal structures of PaCDT (e.g., 5HPQ, shown here) reveals several interactions between the hinge region of chain A with the large domain of chain B that are likely to prevent the wide-open conformation from being sampled. These include packing of Phe195 against the neighbouring subunit, and polar interactions between neighbouring residues. Overlay of the crystal structure of AncCDT-3/L188 (purple, PDB 5JOS) further highlights how the wide-open state is likely to be incompatible with the trimeric arrangement of PaCDT. c The flexibility of the hinge region is known to control the open/closed dynamics of SBPs. Sequence and structural comparison of these regions in AncCDT-3/L188 (purple, PDB 5JOS), AncCDT-5 (green, PDB 6WUP) and PaCDT (white, PDB 5HPQ) highlight that this is an intrinsically flexible region, which is likely affected by mutations.